Translate this page into:
Effect of intravenous dexmedetomidine as an adjuvant to brachial plexus block in upper limb orthopedic surgeries – A systemic review and meta-analysis

-
Received: ,
Accepted: ,
How to cite this article: Joseph P, Ramachandran S, Mohan R, Mary JJF, Ganapathy K, Sanjay P. Effect of intravenous dexmedetomidine as an adjuvant to brachial plexus block in upper limb orthopedic surgeries – A systemic review and meta-analysis. Glob J Health Sci Res. 2024;2:12-7. doi: 10.25259/GJHSR_63_2023
Abstract
Brachial plexus block for upper limb orthopedic surgeries has been widely used for surgical anesthesia and post operative analgesia. Various adjuvants are used to prolong the duration of the nerve block. Systemic dexmedetomidine as an adjuvant to local anesthetics has been shown to prolong the duration of the nerve block in some randomized controlled trials (RCTs) but is far from unanimous in its efficacy. Hence, an updated meta-analysis was planned to assess the efficacy and safety of systemic dexmedetomidine as an adjuvant to local anesthetics in brachial plexus nerve block (BPNB). Objective of the study is to assess the duration of analgesia in patients undergoing upper limb orthopaedic procedures with BPNB and intravenous dexmedetomidine as an adjuvant. Data sources were PubMed, Cochrane, and Google Scholar were systematically searched till July 2023. The meta-analysis included all published studies that investigated the effect of systemic dexmedetomidine on duration of analgesia following BPNB. The data extraction was guided by a predetermined checklist. Analysis was done Using RevMan_5 software, the mean difference for duration of analgesia between the two groups and odds ratio was calculated from the selected studies. The fixed-effects model was used to compare the difference in the duration of analgesia between the two groups. The outcome was prolonged duration of analgesia in patients undergoing upper limb orthopedic procedure where intravenous dexmedetomidine was used as an adjuvant to peripheral nerve blocks. Our meta-analysis currently generates the evidence that intravenous dexmedetomidine administration offers advantages over other drugs in terms of prolonged duration of analgesia.
Keywords
Local anesthesia
Dexmedetomidine
Brachial plexus
Analgesia
Nerve block
INTRODUCTION
Brachial plexus nerve block (BPNB) for the upper limb orthopedic surgeries has been widely used for surgical anesthesia and post-operative analgesia.[1] Various techniques such as continuous perineural catheters, intravenous or perineural dexamethasone, clonidine, midazolam, dexmedetomidine (intravenous and perineural), and adrenaline had been tried to prolong the duration of analgesia postoperatively.[2-5] Perineural administration of adjuvants carries the risk of neurotoxicity and safety is not proven. Dexmedetomidine, a highly selective alpha 2-adrenergic receptor agonist, is widely used perioperatively for its sedative, anxiolytic, and analgesic properties. Systemic dexmedetomidine as an adjuvant to local anesthetics has been shown to prolong the duration of the nerve block in some randomized controlled trials (RCTs) but are far from unanimous in the efficacy.[6-10] Hence, an updated meta-analysis was planned to assess the efficacy and safety of systemic dexmedetomidine as an adjuvant to local anesthetics in BPNB.
MATERIAL AND METHODS
This study protocol was prospectively registered with PROSPERO and conducted with the requirements of the reporting rules in the “Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines”[11] and strictly complied with its specifications. Since this work is a systematic review, the heterogeneity was present within the acceptable range, meta-analysis was performed.
Eligibility criteria
Criteria for included studies were defined as patients undergoing upper limb orthopedic procedure under brachial plexus block. The criteria for the inclusion included,
Patients undergoing upper limb orthopedic procedure under brachial plexus block
RCTs
Studies assessed the duration of analgesia
Studies compared with other drugs or placebo.
Search strategy
The electronic retrieval methods were adopted for the literature retrieval. A comprehensive and systematic research review using combination of Medical Subject Headings (MeSH), controlled vocabulary, and keywords was conducted through various databases, include PubMed, Cochrane, and Google Scholar for studies till 2023. Furthermore, a manual search of reference list of primary trials was conducted from the selected topics and relevant articles were included in the review and analysis.
Study selection
The search results were uploaded into the online systematic review program Rayyan to conduct the study selection.[12] A two-stage screening process were conducted for study selection. Two independent authors (S.R, P.J) performed the literature search and screened the title, abstract, and keywords of all the studies. Screening of abstract and full text was done independently by two authors (S.R, P.J) to select the studies that satisfy the eligibility criteria of our review. Any disagreements or discordances present during the entire selection process were resolved either through consensus or consultation with third author (R.M).
Data extraction and management
The relevant study characteristics for the review were extracted by the first and coauthor independently related to outcome measures from the included studies. Data extraction was guided by a predetermined checklist with the first author’s last name, published year, total sample size, type of surgery, type of nerve block, dose of drug, study design, duration of analgesia, and type of control (placebo or other drugs) [Table 1].
| Study | Year | Number of participants IV group/control group | Type of surgery | Nerve block | Local anesthetic drug and dose | Dexmedetomidine IV dose | Control group | Outcome | Duration of analgesia in IV dexmed group (h) | Duration of analgesia in control group |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdallah et al.[9] | 2016 | 34/32 | Shoulder arthroscopy | Interscalene block | 15 mL ropivacaine 0.5% with adrenaline 1 in 200000 | 0.5 µg/kg | IV saline | Duration of analgesia | 10 | 7 |
| Bao et al.[8] | 2022 | 20/20 | Hand surgery | Mid forearm ulnar radial median nerve block | 0.75% ropivacaine 3 ml each for ulnar radial median nerve | 0.5 µg/kg | IV saline | Duration of analgesia | 11 | 10 |
| Hong et al.[7] | 2018 | 49/47 | Forearm fracture | Supraclavicular block | 25 mL of 1:1 mixture of 1% lidocaine and 0.75% ropivacaine | Loading dose 1 µg/kg Maintenance dose 0.6 µg/kg |
IV midazolam 2 mg | Duration of analgesia | 10 | 7 |
| Kang et al.[10] | 2018 | 18/18 | Shoulder arthroscopy | Interscalene block | 15 mL ropivacaine 0.5% with adrenaline 1 in 200000 | 0.5 µg/kg | IV saline | Duration of analgesia | 11 | 11 |
| Rodrigues et al.[6] | 2020 | 65/65 | Shoulder surgery | Interscalene block | 30 mL 0.5% bupivacaine | 50 µg | IV dexamethasone 4 mg | Duration of analgesia | 16 | 24 |
Second author (P.J) transferred the obtained data into the software Review Manager (RevMan_5.3, Copenhagen: The Nordic Cochrane Center, the Cochrane Collaboration, 2014).[13] Data entry was double-checked for correct entry by the second author (P.J) through comparison of data presented in the review and included the reports.
Outcome measure for the study
The outcome was to assess the duration of analgesia with intravenous dexmedetomidine in patients undergoing upper limb orthopedic procedure.
Quality assessment
Risk of bias by Cochrane was used to assess the risk of bias of the selected articles and the quality review process was monitored. Each article was categorized as follows: “low-risk,” “moderate-risk,” or “high-risk” of bias.
Statistical analysis
A comprehensive qualitative analysis was made. For quantitative meta-analysis, the binomial data were performed using RevMan_5.3. When studies reported multiple arms in single trial, only the relevant arms were included for the analysis. Due to heterogeneity among studies, a logistic-normal-fixed-effect model was conducted. The 95% confidence interval (CI) was performed for study-specific and overall pooled prevalence, respectively. To assess the heterogeny, I2 statistics was used. Significant heterogeny was considered if P < 0.05 or I2 >50% among the studies. Study specific and pooled estimates were graphically represented through forest plot. Sensitivity analysis was done to assess the reliability of the estimate obtained in the meta-analysis.
RESULTS
Study selection and characteristics
A total of 2460 studies were initially retrieved following the removal of duplicates. Of those, five studies met the inclusion criteria. These five articles were ultimately included for the qualitative and quantitative analysis. [Table 1] Of the five articles, three articles compared with saline and one with Midazolam and one with Dexamethasone.[8-10] The PRISMA flowchart for the study selection is available in Figure 1. When using ROB by Cochrane, all five articles had moderate risk of bias [Figures 2 and 3].
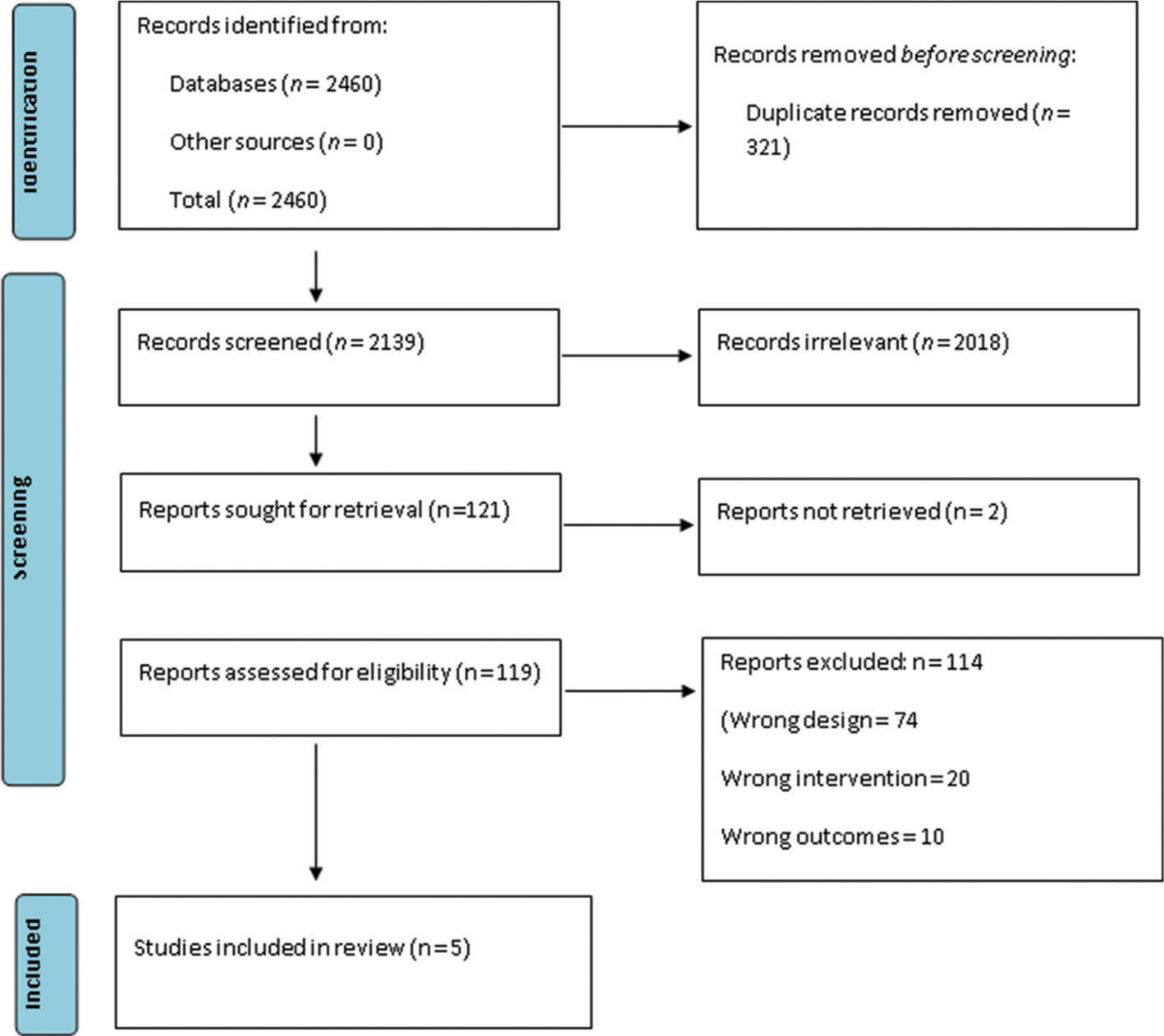
- Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) flow diagram of the study selection process.
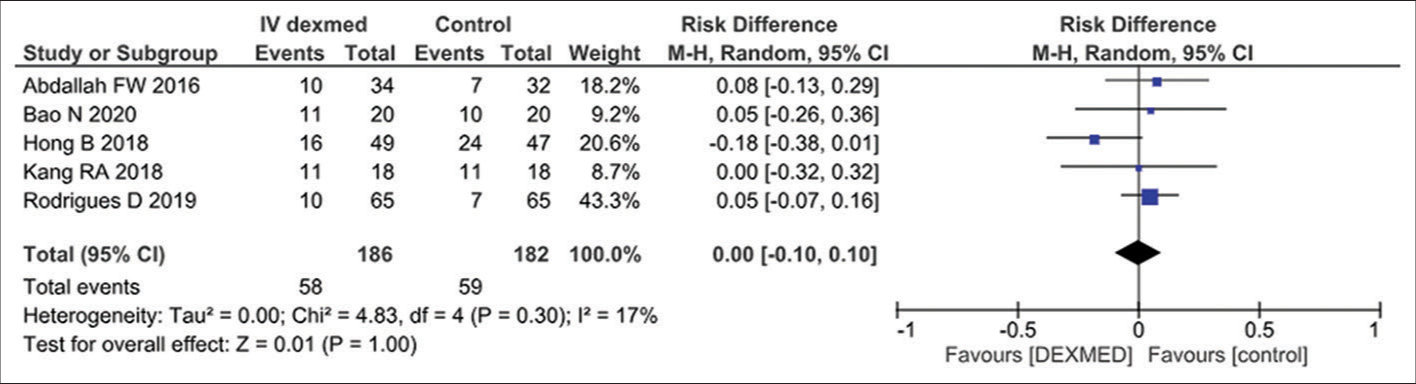
- Effect of dexamedotomidine on brachial plexus nerve block in the included studies. CI: Confidence interval, black diamond: pooled effect.
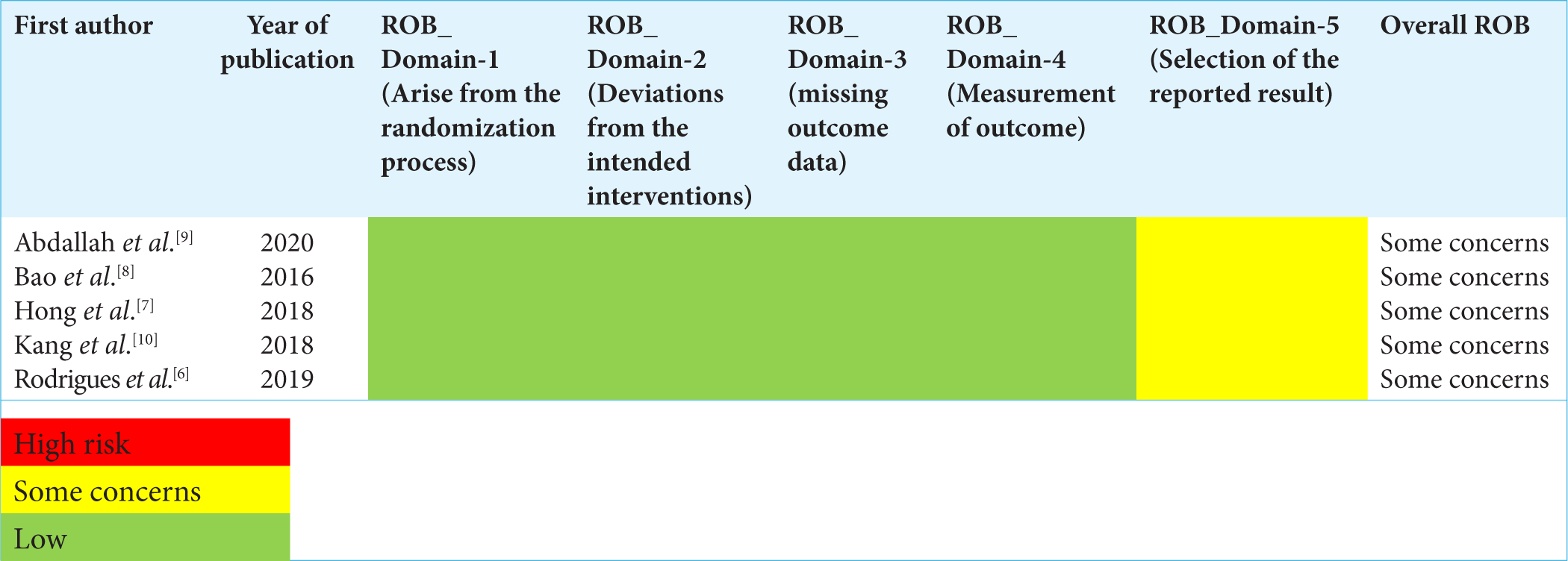
- Risk of Bias (ROB) analysis in the included studies.
Characteristics of the patient
From all five studies included, a total of 186 patients were in the intervention group and 182 patients in the control group who underwent upper limb orthopedic procedure with BPNB. Among them, 186 patients in the intervention group received intravenous dexmedetomidine. Of them, 70 patients in the control group received Intravenous saline, whereas remaining 112 patients received other drugs.[6-10] Overall, the duration of the analgesia ranged between 10 and 16 h in the intervention group and 7 and 24 h in the control group.
Methodological quality of the included studies
The included five studies of the final review were all RCT with other drugs or placebo as control. These articles were published between 2016 and 2020 done in the hospital setting. Among these, one study was triple blinded, and four studies were double blinded.[6-10] [Table 1].
Effect of the intravenous dexmedetomidine
A meta-analysis of five eligible comparative studies to assess the duration of analgesia following peripheral nerve block involving 186 subjects who were exposed to intravenous dexmedetomidine and 182 subjects who were exposed to placebo drug showed an overall significant effect in favor of dexmedetomidine (odds ratio-0.95, 95% CI 0.57–1.58, P = 0.85), as shown in Figure 2. A significant Q statistic (P = 0.35) indicated the presence of heterogeneity (I2 = 9%).
DISCUSSION
Intravenous dexmedetomidine is widely used for sedation and analgesia in critical care unit and procedural sedation in the operating room.[14] The described mechanism underlying the intravenous injection of dexmedetomidine is that it can act on the alpha 2-receptor in the nucleus ceruleus of the brainstem to produce its sedative-hypnotic and antianxiety effects and relieve the patient’s stress.[15] Furthermore, at the level of peripheral nerves, the possible mechanisms of dexmedetomidine as an analgesic adjuvant may be as follows: first, dexmedetomidine suppresses the production of action potentials by C and Aδ fibers, enhances the inhibition of Na+ channels by local anesthetics, and blocks the conduction of excitation;[16] and second, both the activation of inwardly rectifying G1-protein-gated potassium channels and the regulation of entry of calcium through N-type voltage-gated calcium channels are independent of cyclic adenosine monophosphate and protein phosphorylation.[17]
Our meta-analysis showed that intravenous dexmedetomidine as a local anesthetic adjuvant significantly prolonged the duration of analgesia and reduced the analgesic consumption, compared with the placebo group. Administration of intravenous dexmedetomidine as procedural sedation can be used while performing BPNB which can prolong the duration of analgesia postoperatively.
Limitations
Our review has a few limitations. A moderate level of heterogeneity was observed in the measured outcome. The method of operating nerve block was not unified, including ultrasound-guided or nerve stimulator. Finally, the strength of evidence remains limited due to the small number of studies. More high-quality studies are needed to confirm our results.
CONCLUSION
Our meta-analysis currently generates evidence that intravenous dexmedetomidine administration offers advantages over other drugs and placebo in terms of prolonged duration of analgesia.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. P Sanjay, Dr. Reenaa Mohan and Dr. Srinivasan Ramachandran are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Brachial plexus blocks: A review of approaches and techniques. Can J Anaesth. 2007;54:662-74.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided (needle-in-plane) perineural catheter insertion. Reg Anesth Pain Med. 2011;36:261-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:CD011770.
- [CrossRef] [PubMed] [Google Scholar]
- Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks. Anesthesiology. 2009;111:406-15.
- [CrossRef] [PubMed] [Google Scholar]
- Brachial plexus block with midazolam and Bupivacaine improves analgesia. Can J Anesth. 2005;52:822-6.
- [CrossRef] [PubMed] [Google Scholar]
- Analgesic duration of interscalene block after outpatient arthroscopic shoulder surgery with intravenous dexamethasone, intravenous dexmedetomidine, or their combination: A randomized-controlled trial. Can J Anesth. 2021;68:835-45.
- [CrossRef] [PubMed] [Google Scholar]
- Sedation with dexmedetomidine prolongs the analgesic duration of brachial plexus block: A randomized-controlled trial. Anaesth Crit Care Pain Med. 2019;38:231-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dexmedetomidine prolongs the duration of local anesthetics when used as an adjuvant through both perineural and systemic mechanisms: A prospective randomized double-blinded trial. BMC Anesthesiol. 2022;22:176.
- [CrossRef] [PubMed] [Google Scholar]
- IV and Perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016;124:683-95.
- [CrossRef] [PubMed] [Google Scholar]
- Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: A single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med. 2018;43:488-95.
- [CrossRef] [PubMed] [Google Scholar]
- The Prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- Rayyan - AI powered tool for systematic literature reviews. 2021. Available from: https://www.rayyan.ai [Last accessed on 2023 Jul 12]
- [Google Scholar]
- RevMan 5. Available from: https://training.cochrane.org/online-learning/core-software/revman/revman-5-download [Last accessed on 2022 Jul 07]
- [Google Scholar]
- An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43:2064-74.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological modulation of noradrenergic arousal circuitry disrupts functional connectivity of the locus ceruleus in humans. J Neurosci. 2017;37:6938-45.
- [CrossRef] [PubMed] [Google Scholar]
- Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502-11.
- [CrossRef] [PubMed] [Google Scholar]
- Dexmedetomidine: A review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71:1481-501.
- [CrossRef] [PubMed] [Google Scholar]








