Translate this page into:
Safety and efficacy of pre-emptive antifungal therapy versus empirical therapy in patients with febrile neutropenia – A meta-analysis

*Corresponding author: Reena Mohan, Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India. reenaamohan1406@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ranganathan U, Roobhini Sri NSK, Mary J, Mohan R, Ganapathy K, Sanjay P. Safety and efficacy of pre-emptive antifungal therapy versus empirical therapy in patients with febrile neutropenia – A meta-analysis. Glob J Health Sci Res. 2024;2:3-11. doi: 10.25259/GJHSR_65_2023
Abstract
Febrile neutropenia is a life-threatening complication usually seen in cancer chemotherapy patients. Bacterial agents are the most common etiology of sepsis in febrile neutropenia and warrants empirical antibiotic treatment. However, the efficacy of pre-emptive therapy over empirical therapy is debatable. The objectives of this study were to evaluate the efficacy (difference in mortality rate) of pre-emptive antifungal therapy in patients with febrile neutropenia compared to empirical antifungal therapy and to evaluate the safety (antifungal exposure, adverse effects, and duration of hospital stay) of pre-emptive antifungal therapy. The data source used for the study is only PubMed. Only full-text articles in English language since the year 2000 were included. Unpublished studies will not be sought. Searches will be re-run before analysis. Data extraction was guided by a predetermined checklist. Using RevMan 5 software, the effect of intervention is null (95% CI 0.66–1.91, P = 0.57)]. An insignificant Q statistic (P > 0.66) indicates the absence of heterogeneity (I2 = 0%) as there is not much difference in the mortality rates between two groups. Data analyses were performed from June 2023 to August 2023. The primary outcome is an insignificant Q statistic (P > 0.66) indicates the absence of heterogeneity (I2 = 0%) as there is not much difference in the mortality rates between two groups. Hence, pre-emptive therapy can be considered in place of empirical therapy to avoid over treatment with antifungal agents in patients with febrile neutropenia. A meta-analysis of five eligible comparative studies involving 588 subjects who had pre-emptive antifungal therapy and 587 subjects who had empirical therapy signifies the effect of intervention is null (95% CI 0.66–1.91, P = 0.57). An insignificant Q statistic (P > 0.66) indicates the absence of heterogeneity (I2 = 0%) as there is not much difference in the mortality rates between two groups. Hence, pre-emptive therapy can be considered in place of empirical therapy to avoid over treatment with antifungal agents in patients with febrile neutropenia. This systematic review and meta-analysis demonstrated that pre-emptive therapy can be considered in place of empirical therapy to avoid over treatment with antifungal agents in patients with febrile neutropenia. Trial Registration: PROSPERO receipt number-443707.
Keywords
Febrile neutropenia
Antifungal therapy
Pre-emptive antifungal therapy and empirical antifungal therapy
INTRODUCTION
Febrile neutropenia is a life-threatening complication usually seen in cancer chemotherapy patients.[1,2] Bacterial agents are the most common etiology of sepsis in febrile neutropenia and warrants antibiotic treatment. Febrile neutropenic cancer patients are also at increased risk for invasive fungal disease (IFD) associated with fatal outcomes. The common fungal agents associated with IFD are Candida species, Aspergillus, Cryptococcus, and Pneumocystis.[3] Targeted therapy is not always feasible as cultures take time and anti-fungal susceptibility cannot always be done due to lack of resources and guidelines. Classically, empirical antifungal therapy is recommended for patients with persisting fever for more than three days after broad-spectrum antimicrobial therapy.[4,5] Empirical antifungal therapy, though initiated as a life-saving measure can lead to low specificity, over-treatment of the patients, antifungal resistance and higher medical expenses.[6] Pre-emptive antifungal therapy is an alternate evidence-based approach to avoid overtreatment. However, the efficacy of preemptive therapy over empirical therapy is debatable, and hence, this meta-analysis aims to evaluate the safety and efficacy of pre-emptive antifungal therapy versus empirical therapy in patients with febrile neutropenia.
MATERIAL AND METHODS
This study protocol was prospectively registered with PROSPERO and conducted with the requirements of the reporting rules in the “Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines”[7] and strictly complied with its specifications. Since this work is a systematic review, the heterogeneity was present within the acceptable range, meta-analysis was performed.
Eligibility criteria
All patients with febrile neutropenia receiving pre-emptive or empirical antifungal therapy were included in the study.
The criteria for the inclusion included,
All patients with febrile neutropenia receiving antifungals as pre-emptive or empirical therapy
Clinical criteria or serological tests like (galactomannan or 1, 3, beta-D-glucan assay) guided pre-emptive therapy were considered.
Studies that assessed the efficacy and safety of preemptive and empirical therapy antifungal therapy in febrile neutropenic patients
Randomized controlled trials (RCTs).
Search strategy
The electronic retrieval methods were adopted for the literature retrieval. A comprehensive and systematic research review using a combination of Medical Subject Headings (MeSH), controlled vocabulary, and keywords was conducted through PubMed for studies from the year 2000 to 2023. The full search strategy is available in Table 1.
| Search number | Query | Sort by | Filter | Search details | Results | Time |
|---|---|---|---|---|---|---|
| 1. | ((Febrile neutropenia) OR (“Fever neutropenia”)) OR (“Neutropenic fever”) | “febrile neutropenia”[MeSH Terms] OR (“febrile”[All Fields] AND “neutropenia”[All Fields]) OR “febrile neutropenia”[All Fields] OR “Fever neutropenia”[All Fields] OR “Neutropenic fever”[All Fields] | 11,180 | 02:32:42 | ||
| 2. | Preemptive antifungal therapy | (“preemptive”[All Fields] OR “preemptively”[All Fields]) AND (“antifungal agents”[Pharmacological Action] OR “antifungal agents”[MeSH Terms] OR (“antifungal”[All Fields] AND “agents”[All Fields]) OR “antifungal agents”[All Fields] OR “antifungal”[All Fields] OR “antifungals”[All Fields] OR “antifungic”[All Fields] OR “antifungical”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “therapy s”[All Fields] OR “therapys”[All Fields]) | 545 | 02:33:30 | ||
| 3. | Empirical antifungal therapy | (“empiric”[All Fields] OR “empirical”[All Fields] OR “empirically”[All Fields] OR “empirics”[All Fields]) AND (“antifungal agents”[Pharmacological Action] OR “antifungal agents”[MeSH Terms] OR (“antifungal”[All Fields] AND “agents”[All Fields]) OR “antifungal agents”[All Fields] OR “antifungal”[All Fields] OR “antifungals”[All Fields] OR “antifungic”[All Fields] OR “antifungical”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “therapy s”[All Fields] OR “therapys”[All Fields]) | 2,113 | 02:45:53 | ||
| 4. | #1 AND #2 AND #3 | (“febrile neutropenia”[MeSH Terms] OR (“febrile”[All Fields] AND “neutropenia”[All Fields]) OR “febrile neutropenia”[All Fields] OR “Fever neutropenia”[All Fields] OR “Neutropenic fever”[All Fields]) AND ((“preemptive”[All Fields] OR “preemptively”[All Fields]) AND (“antifungal agents”[Pharmacological Action] OR “antifungal agents”[MeSH Terms] OR (“antifungal”[All Fields] AND “agents”[All Fields]) OR “antifungal agents”[All Fields] OR “antifungal”[All Fields] OR “antifungals”[All Fields] OR “antifungic”[All Fields] OR “antifungical”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “therapy s”[All Fields] OR “therapys”[All Fields])) AND ((“empiric”[All Fields] OR “empirical”[All Fields] OR “empirically”[All Fields] OR “empirics”[All Fields]) AND (“antifungal agents”[Pharmacological Action] OR “antifungal agents”[MeSH Terms] OR (“antifungal”[All Fields] AND “agents”[All Fields]) OR “antifungal agents”[All Fields] OR “antifungal”[All Fields] OR “antifungals”[All Fields] OR “antifungic”[All Fields] OR “antifungical”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “therapy s”[All Fields] OR “therapys”[All Fields])) | 39 | 02:51:14 |
Study selection
The search results were uploaded into the online systematic review program Rayyan to conduct the study selection.[8] A two-stage screening process was conducted for study selection. Two independent authors (U.R, R.NSK) performed the literature search and screened the title, abstract, and keywords of all the studies. Screening of abstract and full text was done independently by two authors (U.R, R.NSK) to select the studies that satisfy the eligibility criteria of our review. Any disagreements or discordances present during the entire selection process were resolved either through consensus or consultation with a third author (R.M). If conflicts arose between reviewers, the fourth reviewer (J.F) moderated a discussion to come to a joint decision.
Data extraction and management
The relevant study characteristics for the review were extracted by the first and coauthor independently related to outcome measures from the included studies. Data extraction was guided by a predetermined checklist with the first author’s last name, published year, total sample size, gender, study design, participants’ age, strategy for deciding pre-emptive antifungal therapy, major intended outcome (difference in mortality), and other study outcomes (duration of anti-fungal therapy, side effects related to use of antifungal therapy, and duration of hospital stay) which were extracted [Tables 2 and 3].
| First author | Year of publication | Study setting | Study design | Blinding | Study period | Study population | Sampling strategy | Intervention group | Type of comparator | Age (Median, IQR or Mean±SD) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||||
| Cordonnier et al.[11] |
2009 | Hospital | Prospective, randomized, open-label noninferiority trial | Double blind | 2003–2006 | Adults | Randomisation | Preemptive antifungal | Empirical antifungal | 52.1 (14.1) | 52 (13.5) |
| Kanda et al.[12] |
2020 | Hospital | Randomized trial | Single blind | 2013–2017 | Adults | Randomisation | Preemptive antifungal | Empirical antifungal | 55 (20–77) | 56 (19-78) |
| Santolaya et al.[13] |
2018 | Hospital | Prospective, multicentre, randomized clinical trial | Double blind | 2013–2016 | children and adolescents | Randomisation | Preemptive antifungal | Empirical antifungal | 7 (3–11) | 6 (4–12) |
| Tan et al.[14] | 2011 | Hospital | Prospective, randomized, non-blinded study | Non-blind | 2006–2007 | Adults | Randomisation | Preemptive antifungal | Empirical antifungal | 44 (17–67) | 45 (16–77) |
| Yuan et al.[15] | 2016 | Hospital | Randomized trial | Not mentioned | 2013–2014 | Adults | Randomisation | Preemptive antifungal | Empirical antifungal | 38 (18–81) | 38 (18–77) |
IQR: Interquartile range, SD: Standard deviation
| First author | Mortality due to IFD n(%) | Days of antifungal therapy (Median, IQR or Mean±SD) |
Days of hospitalization (Median, IQR or Mean±SD) |
|||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Cordonnier et al.[11] | 3 (2.1) | 0 (0) | 4.5 (7.3) | 7 (8.3) | 30.3 (10.2) | 30.3 (10.5) |
| Kanda et al.[12] | 15 (3.8) | 16 (3.6) | ||||
| Santolaya et al.[13] | 2 (3) | 2 (3) | 11 (7–16) | 6 (3–13) | 17 (13–22) | 19 (14–23) |
| Tan et al.[14] | 4 (14.8) | 4 (16) | ||||
| Yuan et al.[15] | 3 (2.3) | 1 (0.7) | 13.8 (4.7) | 20 (4.7) | 32.7 (9.3) | 34 (11.3) |
IFD: Invasive fungal disease, IQR: Interquartile range, SD: Standard deviation
Second author (R.NSK) transferred the obtained data into the software Review Manager (RevMan_5.4, Copenhagen: The Nordic Cochrane Center, the Cochrane Collaboration, 2014).[9] Data entry was double-checked for correct entry by the first author (U.J.) through a comparison of data presented in the review and included the reports.
Outcome measure for the study
The primary outcome was to assess any effect on mortality in pre-emptive antifungal arm compared to empirical antifungal arm in febrile neutropenic patients and the secondary outcome was to evaluate any effect on the duration of hospital stay, days of antifungal usage, and adverse effects associated with antifungal agents in the empirical arm compared to pre-emptive arm.
Quality assessment
The revised Cochrane risk-of-bias tool for randomized trials[10] was used to assess the risk of bias of the selected articles and the quality review process was monitored. Each article was categorized as follows: “low-risk,” “moderate-risk,” or “high-risk” of bias [Table 4].
| First author | Year of publication | ROB_ Domain-1 (Arise from the randomization process) | ROB_Domain-2 (Deviations from the intended interventions) | ROB_ Domain-3 (missing ourcome data) | ROB_ Domain-4 (Measurement of outcome) | ROB_ Domain-5 (Selection of the reported result) | Overal ROB |
|---|---|---|---|---|---|---|---|
| Cordonnier et al.[11] | 2009 | Low | |||||
| Kanda et al.[12] | 2020 | Some concerns | |||||
| Santolaya et al.[13] | 2018 | Low | |||||
| Tan et al.[14] | 2011 | Some concerns | |||||
| Yuan et al.[15] | 2016 | High risk | |||||
| High risk | |||||||
| Some concerns | |||||||
| Low |
ROB: Risk of bias
Statistical analysis
A comprehensive qualitative analysis was made. For quantitative meta-analysis, the binomial data were performed using RevMan_5.4.[9] When studies reported multiple arms in single trial, only the relevant arms were included for the analysis. Due to heterogeneity among studies, a logistic-normal-random-effect model was conducted. The 95% confidence interval (CI) was performed for study-specific and overall pooled prevalence, respectively. To assess the heterogeny, I2 statistics was used. Significant heterogeny was considered if P < 0.05 or I2 >50% among the studies.
Subgroup analysis was performed to assess the heterogeneity and potential confounding for studies. Study specific and pooled estimates were graphically represented through forest plots for both combined and subgroup analysis. Publication bias was assessed and graphically represented by funnel plot and asymmetry of the plot was tested using Egger’s test. Sensitivity analysis was done to assess the reliability of the estimate obtained in the meta-analysis.
RESULTS
Study selection and characteristics
A total of 39 studies were initially retrieved. Primary screening excluded 19 studies as they had wrong study design or outcome. Of the remaining 20 studies, secondary screening excluded 15 studies due to wrong study design or outcome. Thus, five articles were included for the qualitative and quantitative analysis [Table 1].[11-15]
Of the five articles, one article had high risk of bias, two articles had low risk of bias, and two articles had moderate risk of bias according to the revised Cochrane risk-of-bias tool for randomized trials [Table 4]. The PRISMA flowchart for the study selection is available in Figure 1. All the five studies were hospital based. Of the five articles, four had adult population,[11,12,14,15] while one study had children and adolescent population.[13]
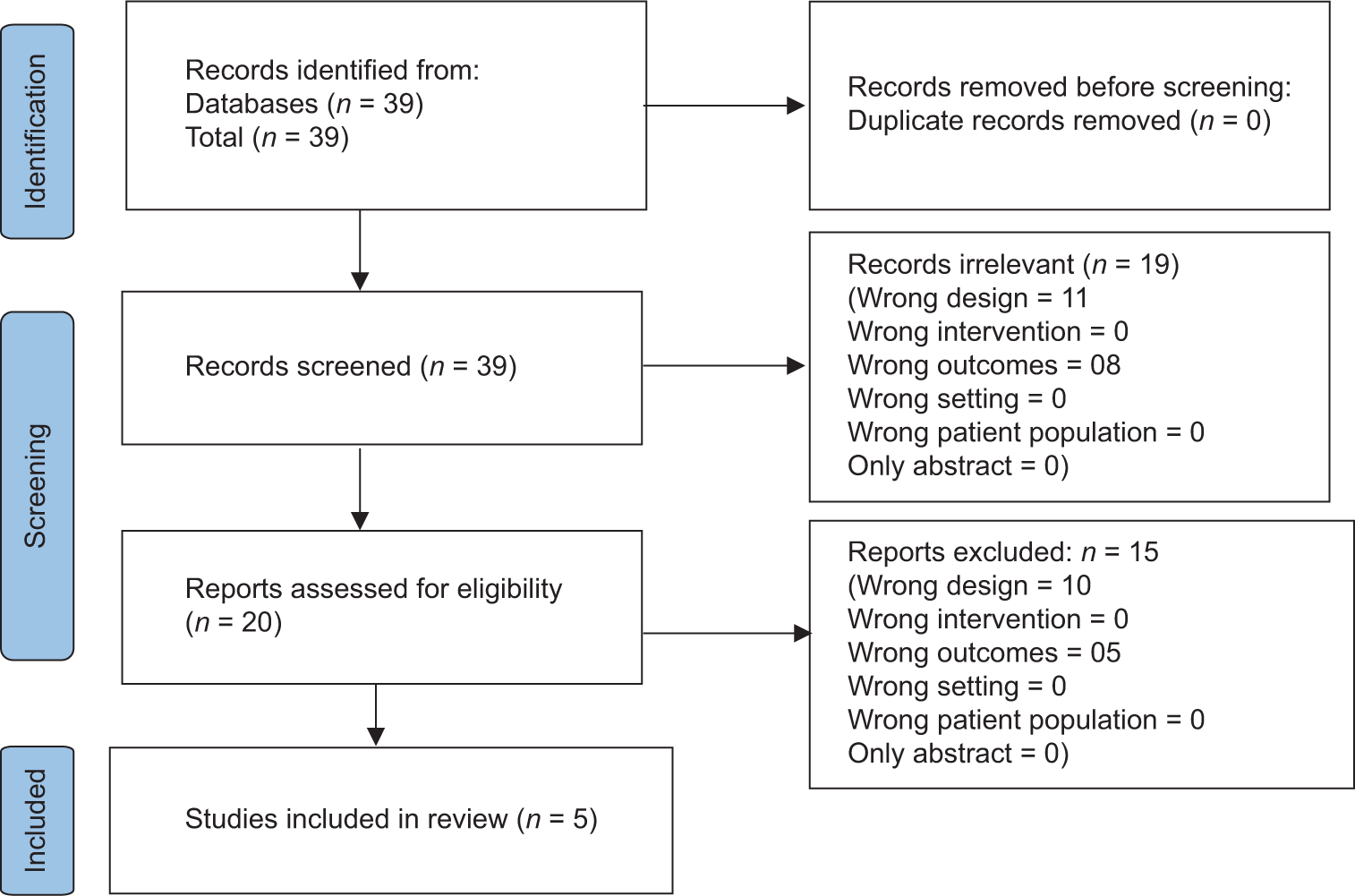
- Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) flow diagram of the study selection process.
Characteristics of the patient and the criteria used to start antifungals pre-emptively
From all five studies included, a total of 588 patients in the intervention group and 587 patients on the control group who had febrile neutropenia received antifungals as preemptive and empirical, respectively. The age of the overall cohorts included in this study ranged from 3 to 81 years of age. The criteria used to start antifungals pre-emptively in these studies are provided in Table 5.
| S. No. | Study | Pre-emptive methods |
|---|---|---|
| 1. | Cordonnier et al.[11] | Any time after 4 days of fever and antibacterial treatment:
|
| 2. | Kanda et al.[12] | On 4th day of persisted fever and antibacterial treatment
|
| 3. | Santolaya et al.[13] | Persistent fever and ANC<500/mm3 were accompanied by any of the following findings suggesting IFD
|
| 4. | Tan et al.[14] |
|
| 5. | Yuan et al.[15] | Any time after 4 days of fever and antibacterial treatment:
|
CT: Computed tomography, CNS: Central nervous system, IFD: Invasive fungal disease, IFI: Invasive fungal infection, ANC: Absolute neutrophil count, RCT: Randomized controlled Trials, ELISA: Enzyme linked immunosorbent assay
Methodological quality of the included studies
The included five studies for the final review were all RCT with empirical antifungal therapy as control. These articles were published between 2009 and 2020 done in the hospital setting. Among these, two trails were double blinded,[11,13] one was a single blinded study,[12,14] one study was not blinded, while one study has not reported the blinding[15] [Table 1].
EFFECT ON MORTALITY BETWEEN PREEMPTIVE AND EMPIRICAL ARM
A meta-analysis of five eligible comparative studies involving 588 subjects who had pre-emptive antifungal therapy and 587 subjects who had empirical therapy signifies the effect of intervention is null (95% CI 0.66 to 1.91, P = 0.57) as shown in Figure 2. An insignificant Q statistic (P > 0.66) indicates the absence of heterogeneity (I2 = 0%) as there is not much difference in the mortality rates between two groups. Hence, pre-emptive therapy can be considered in place of empirical therapy to avoid over treatment with antifungal agents in patients with febrile neutropenia.
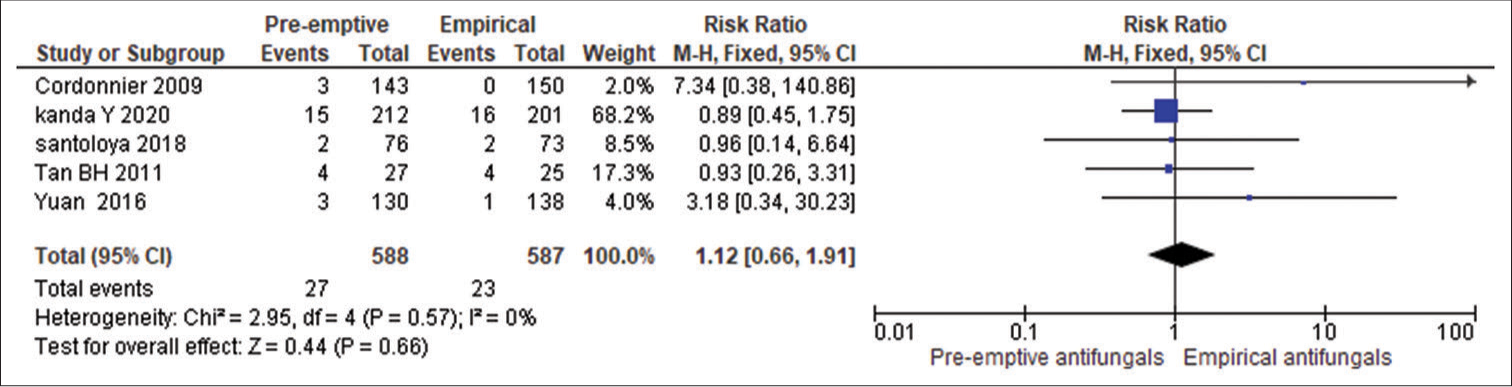
- Effect on mortality in pre-emptive antifungal and empirical antifungal treatment. CI: confidence interval, M-H: Mantel-Haenszel.
EFFECT ON DURATION OF HOSPITAL STAY
Of the five RCT included in the meta-analysis, effect on hospital stay data was available for 3 studies.[11,13,15] In the study by Cordonnier et al., there was no difference in the mean duration of hospital stay in both the groups, while Yuan et al., showed reduced mean duration of stay in preemptive group compared to empirical group. The study by Santolaya et al., also showed reduced median duration of stay in preemptive group compared to empirical group [Table 3].[11,13,15]
EFFECT ON DAYS OF ANTIFUNGAL THERAPY
Of the five RCT included in the meta-analysis, effect on hospital stay data was available for 3 studies.[11,13,15] The study by Cordonnier et al. and Yuan et al., showed reduced mean duration of antifungal therapy in preemptive group compared to empirical group while Santolaya et al. showed high median duration of stay in preemptive group compared to empirical group [Table 3].[11,13,15]
DISCUSSION
Cancer patients with neutropenia are at increased risk for developing IFD. Early diagnosis and treatment of IFD is crucial and life-saving. IFDs are usually identified based on clinical, radiological, histopathology, and microbiology (fungal culture) studies. Recently, non-culture-based serological tests such as galactomannan and 1,3 beta-D glucan assays provide highly sensitive and rapid results for IFD.[16,17] Despite these advances, patients are usually started on empirical antifungal therapy when there is neutropenia and fever after 3 days of antibacterial treatment. However, the disadvantage of this strategy is that patient without IFD might receive the antifungal treatment, leading to increased cost of treatment, prolonged hospital stay (related to side effects of antifungals), and emergence of antifungal resistance. Hence, antifungals should be initiated only when there is evidence for IFD. Although pre-emptive treatment may alleviate these drawbacks, the approach’s influence on mortality rates is uncertain.
Overall, in the five trials included in this meta-analysis, it is demonstrated that patients receiving preemptive antifungal did not have any significant difference in mortality compared to the empirical group due to IFD.
In addition, it is also shown that the median duration of stay in the preemptive group is less compared to empirical group. Furthermore, two studies showed a reduced mean duration of antifungal therapy preemptive group compared to the empirical group while one study gave conflicting results.
Despite significant information demonstrated above, our meta-analysis has its own limitations. Only limited RCTs were analyzed in our study, contributing to a very small sample size, limiting its extrapolation to the general population.
CONCLUSION
IFD is a fatal condition in febrile neutropenic cancer patients. The classical approach of empirical antifungal therapy for IFD though life-saving can lead to over treatment in patients without IFD and complications related to it. Analysis of completed clinical trials to date shows no significant difference in mortality when adapting preemptive treatment approach. Furthermore, preemptive approach reduces days of hospital stay and days of antifungal exposure. Hence, we propose preemptive antifungal treatment for IFD, where feasible over empirical treatment in the case of febrile neutropenic cancer patients. We also propose further exploration involving more RCTS to strengthen this evidence in the future.
Authors’ contributions
Drs. U.R and R.NSK had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Drs. R.M. and J.F. contributed equally. Drs K.G and S.P were the co-seniors in the study. Concept and Design: UR and R.NSK. Acquisition of Data: U.R and R.NSK. Analysis or Interpretation of Data: R.M. and J.F. Drafting of the Manuscript: U.R, R.NSK and R.M. Critical Revision of the Manuscript for Important Intellectual Content: K.G. and S.P. Statistical analysis: J.F. Administrative, Technical, or Material Support: K.G and S.P. Supervision: K.G and S.P.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. Udhaya Sankar Ranganathan, Dr. Reenaa Mohan and Dr. P. Sanjay are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: An autopsy study over a 15-year period (1989-2003) Haematologica. 2006;91:986-9.
- [Google Scholar]
- Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis. 2009;48:265-73.
- [CrossRef] [PubMed] [Google Scholar]
- Common invasive fungal diseases: An overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly. 2016;146:w14281.
- [CrossRef] [PubMed] [Google Scholar]
- 2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730-51.
- [CrossRef] [PubMed] [Google Scholar]
- Empirical antifungal therapy. Int J Antimicrob Agents. 2004;23:105-12.
- [CrossRef] [PubMed] [Google Scholar]
- The use and efficacy of empirical versus pre-emptive therapy in the management of fungal infections: The HEMA e-Chart Project. Haematologica. 2011;96:1366-70.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- Rayyan - AI powered tool for systematic literature reviews. 2021. Available from: https://www.rayyan.ai [Last accessed on 2023 Jul 12]
- [Google Scholar]
- RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- [CrossRef] [PubMed] [Google Scholar]
- Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: A randomized, controlled trial. Clin Infect Dis. 2009;48:1042-51.
- [CrossRef] [PubMed] [Google Scholar]
- D-index-guided early antifungal therapy versus empiric antifungal therapy for persistent febrile neutropenia: A randomized controlled noninferiority trial. J Clin Oncol. 2020;38:815-22.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of pre-emptive versus empirical antifungal therapy in children with cancer and high-risk febrile neutropenia: A randomized clinical trial. J Antimicrob Chemother. 2018;73:2860-6.
- [CrossRef] [PubMed] [Google Scholar]
- Galactomannan-guided preemptive vs. empirical antifungals in the persistently febrile neutropenic patient: A prospective randomized study. Int J Infect Dis. 2011;15:e350-6.
- [CrossRef] [PubMed] [Google Scholar]
- Preemptive antifungal therapy for febrile neutropenic hematological malignancy patients in China. Med Sci Monit. 2016;22:4226-32.
- [CrossRef] [PubMed] [Google Scholar]
- Serum (1→3)-β-D-glucan and galactomannan levels in patients with cystic fibrosis: A retrospective cohort study. BMC Pulm Med. 2018;18:52.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: A prospective validation. Blood. 2001;97:1604-10.
- [CrossRef] [PubMed] [Google Scholar]







