Translate this page into:
Effect of azithromycin administration in cases of acute bronchiolitis – A systematic review and meta-analysis

*Corresponding author: Kanimozhi Thandapani, Department of Pediatrics, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India. drkani88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tamilarasan P, Thandapani K, Mary J, Mohan R, Ganapathy K. Effect of azithromycin administration in cases of acute bronchiolitis – A systematic review and meta-analysis. Glob J Health Sci Res 2023;1:69-75.
Abstract
Acute Bronchiolitis is the most common viral lower respiratory tract infection in infants requiring hospitalization. Management is usually symptomatic and supportive with no specific treatment options. Although viral, Azithromycin by its anti-inflammatory properties might play a role in improving the clinical outcome. The objective was to assess the effect of azithromycin on length of hospital stay and duration of oxygen requirement in cases of acute bronchiolitis. Data sources such as PubMed and Google Scholar were systematically searched till June 2023. The meta-analysis included all published, randomized controlled trials that investigated the efficacy of Azithromycin over placebo in infants <24 months with acute bronchiolitis. Data extraction was guided by a predetermined checklist. Using RevMan 5 software, the mean length of hospital stay and duration of oxygen administration were pooled from the selected studies. The fixed-effect model was used to compare the length of hospital stay and the duration of oxygen administration in the Azithromycin and placebo group. Data analyses were performed in July 2023. The primary outcome was a comparison of the length of hospital stay in infants with Bronchiolitis receiving either azithromycin (intervention) or placebo (control). The secondary outcome was the assessment of the duration of oxygen requirement in both intervention and control groups.The initial search yielded 601 records of which 68 articles underwent full-text evaluation, which identified four articles and a total of 571 patients that were included. The findings did not favor the usage of azithromycin antibiotic in treatment of acute bronchiolitis (Mean deviation = 1.21, 95% CI 0.53–2.78, P = 0.80). An insignificant Q statistic (P = 0.001) indicated the absence of heterogeneity (I2 = 0%). Length of hospital stay showed an overall insignificant effect as the length of stay is almost similar in both drugs (OR = 1.04 95% CI 0.49–2.23, P = 1.00). An insignificant Q statistic (P = 1.00) indicated the absence of heterogeneity (I2 = 0%).
Keywords
Azithromycin
Bronchiolitis
Infants
Macrolide
Oxygen requirement
INTRODUCTION
Acute bronchiolitis is the most common lower respiratory tract infection in infants and young children. The etiology is viral, most common being respiratory syncytial virus. It often results in hospitalization of children.[1] Bronchiolitis in early infancy is a major risk factor for recurrent wheezing and asthma in childhood.[2] Treatment is usually symptomatic including humidified oxygen and hydration. Although hypertonic saline and racemic epinephrine are commonly used in hospitalized infants, studies on their efficacy show mixed results. Other interventions such as bronchodilators, mucolytics, inhaled corticosteroids, oral antibiotics, and antiviral drugs have been found to be ineffective.[1] Despite bronchiolitis being a viral infection, antibiotics are widely prescribed for possible secondary bacterial infections such as pneumonia and septicemia.[3,4] Azithromycin is a macrolide antibiotic with additional anti-inflammatory and immunomodulatory effects. It targets neutrophilic airway inflammation based on mouse models of bronchiolitis. In infants, this was demonstrated by reduction in IL-8 levels of nasal lavage.[2] This explains the poor response to drugs like corticosteroids which have minimal action on noneosinophilic inflammation which is the predominant pattern in bronchiolitis. Even other effects of azithromycin have also been studied. It has been found to induce interferon-stimulated genes in rhinovirus-infected bronchial epithelial cells. It can also reduce nasopharyngeal bacterial loads and may transiently reduce the risk of acute lower respiratory infection.[5] It has direct action against respiratory bacteria, especially Mycoplasma and Chlamydia species. Studies have also shown beneficial effects of azithromycin in symptoms of chronic lung disorders such as cystic fibrosis and bronchiectasis due to reduction in neutrophilic inflammation. It has also been found to reduce the number of wheezing episodes after bronchiolitis.[2,6,7] There have been studies regarding the efficacy of azithromycin during the episodes of viral bronchiolitis, but these have provided mixed results.[4,5,8,9] Hence, we planned to perform a meta-analysis to determine whether azithromycin has any beneficial effect in reducing the length of stay or duration of oxygen administration in such children.
MATERIAL AND METHODS
This study protocol was prospectively registered with PROSPERO and conducted with the requirements of the reporting rules in the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines” and strictly complied with its specifications. Since this work is a systematic review, the heterogeneity was present within the acceptable range, meta-analysis was performed.
Eligibility criteria
The criteria for the inclusion included,
Infants <24 months of age admitted with acute bronchiolitis and receiving either azithromycin or placebo
Oral azithromycin as intervention in any dose or duration
Outcome indicators: Length of hospital stay and duration of oxygen administration
Double-blinded and randomized controlled trials (RCT) with two groups randomized to receive azithromycin or placebo
Studies in English language.
Exclusion criteria
The following criteria were excluded from the study:
Other study designs such as case–control study, cross-sectional study/observational study, and open-label RCT
Studies with incomplete data
Animal studies.
Search strategy
Electronic retrieval methods were adopted for literature retrieval. A comprehensive and systematic research review using a combination of Medical Subject Headings, controlled vocabulary, and keywords was conducted through databases including PubMed and Google Scholar for studies till 2023. The keyword used was “Bronchiolitis,” “Azithromycin,” “infants,” “children,” and “Randomized control trial.” Furthermore, a manual search of the reference list of primary trials was conducted from the selected topics, and relevant articles were included in the review and analysis.
Study selection
The search results were uploaded into the online systematic review program Rayyan to conduct the study selection. A two-stage screening process was conducted for study selection. Two independent authors (P.T, K.T) performed the literature search and screened the title, abstract, and keywords of all the studies. Screening of abstract and full text was done independently by two authors (P.T, K.T) to select the studies which satisfy the eligibility criteria of our review. Any disagreements or discordances present during the entire selection process were resolved either through consensus or consultation with the third author (R.M). If conflicts arose between reviewers, the fourth and fifth reviewers (J.F.M, K.G) moderated a discussion to come to a joint decision.
Data extraction and management
The relevant study characteristics for the review were extracted by the first and coauthor independently related to outcome measure from the included studies. Data extraction was guided by a predetermined checklist with first author’s last name, published year, total sample size, gender, study design, duration of intervention, participant’s age, infants <24 months with bronchiolitis, type of intervention (placebo or Azithromycin), length of hospital stay, and duration of oxygen requirement which were extracted.
The first author (P.T) transferred the obtained data into the software Review Manager (RevMan_5.3). Data entry was double-checked for correct entry by the second author (K.T) through a comparison of data presented in review and included the reports.
Outcome measure for the study
The primary outcome was the comparison of the length of hospital stay in infants with bronchiolitis receiving either azithromycin (intervention) or placebo (control). Secondary outcome was assessment of duration of oxygen requirement in both intervention and control groups.
Quality assessment
The revised Cochrane risk-of-bias tool for randomized trials (RoB 2) was used to assess the risk of bias of the selected articles and the quality review process was monitored. Each study was categorized as follows: “low risk,” “some concerns,” or “high risk” of bias.
Statistical analysis
A comprehensive qualitative analysis was made. For quantitative meta-analysis, the binomial data were performed using RevMan_5.3. When studies reported multiple arms in single trial, only the relevant arms were included for the analysis. Due to heterogeneity among studies, a logistic-normal random-effect model was conducted. The 95% Confidence Interval (CI) was performed for study-specific and overall pooled prevalence, respectively. To assess the heterogeny, I2 statistics was used. Significant heterogeny was considered if P < 0.05 or I2 > 50% among the studies.
RESULTS
Study selection and characteristics
A total of 601 studies were initially retrieved following the removal of duplicates. On screening, 537 studies were deemed irrelevant to our review. The remaining 64 were assessed for eligibility. Of those, four studies met the inclusion criteria and were ultimately included in the qualitative and quantitative analysis. [Figure 1] illustrates the PRISMA flowchart for the study selection.

- Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram of the study selection process.
When using the Cochrane risk-of-bias tool, two studies had a low risk of bias and two studies had some concerns. The major limitation was the small sample size in two studies. Baseline characteristics were found to be similar in both intervention and control groups in all studies. Although there were variations in length of hospital stay and duration of oxygen requirement in the intervention group as compared to placebo, the results were not statistically significant in all four studies.
Characteristics of the study population
From all four studies included, a total of 276 patients were in the intervention group and 295 patients were in the control group. The mean age for the overall cohorts included in this study ranged from 3 months to 5.7 months. All the studies used azithromycin for intervention group and placebo for the control group. The duration of the intervention was single dose in a study by McCallum et al., 3 days in study by Kneyber et al., 7 days in the study by Pinto et al., and 3 weekly doses in a study by McCallum et al.[4,5,8,9] [Tables 1 and 2] depict the characteristics of the study population and their outcome data.
| Author | Journal | Study setting | Study design | Blinding | Study period | Study population | Sampling strategy | Intervention group | Type of comparator | Type of analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Kneyber et al. 2008 | Pediatric pulmonology | Hospital | RCT | Double blind | 3 years | Children | Randomization | Azithromycin | Placebo | ITT |
| Pinto et al. 2012 | The Journal of pediatrics | Hospital | RCT | Double blind | 3 years | Children | Randomization | Azithromycin | Placebo | PP |
| McCallum et al. 2013 | PLoS One | Hospital | RCT | Double blind | 3 years | Children | Randomization | Azithromycin | Placebo | PP |
| McCallum et al. 2015 | Frontiers in pediatrics | Hospital | RCT | Double blind | 3 years | Children | Randomization | Azithromycin | Placebo | Not mentioned |
RCT: Randomized controlled trials, ITT: Intention to treat, PP: Per protocol analysis
| Author | Age (months) | Sample size | Length of hospital stay (days) | Duration of oxygen administration (days) | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Kneyber MC 2008 | 3 | 3.5 | 32 | 39 | 5 | 6 | 4 | 3 |
| Pinto LA 2012 | 3.08 | 3.12 | 88 | 96 | 5 | 5 | 4 | 4 |
| Mc Callum 2013 | 5.3 | 5 | 50 | 47 | 2 | 2 | 1 | 2 |
| Mc Callum 2015 | 5.7 | 5.6 | 106 | 113 | 2 | 2 | 2 | 1 |
Methodological quality of the included studies
The included four studies of the final review were all double-blinded RCT with placebo as control. These articles were published between 2008 and 2015 done in the hospital setting.
Length of hospital stay
A meta-analysis of four eligible comparative studies involving 276 subjects exposed to azithromycin and 295 subjects exposed to placebo drug did not favor usage of azithromycin antibiotics in treatment of acute bronchiolitis (MD = 1.21, 95% CI 0.53–2.78, P = 0.80), as shown in [Figure 2]. An insignificant Q statistic (P > 0.001) indicated the absence of heterogeneity (I2 = 0%).
- Length of hospital stay in azithromycin group versus placebo group.
Duration of oxygen requirement
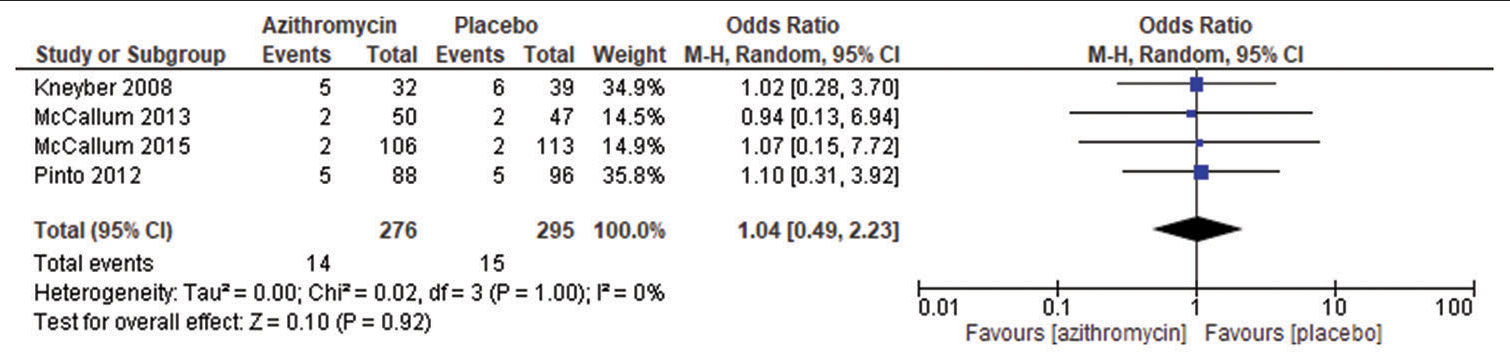
A meta-analysis of subgroup analysis four eligible comparative studies involving 276 subjects exposed to azithromycin and 295 subjects exposed to placebo drug and their associated length of hospital stay showed an overall insignificant effect as the length of stay is almost similar in both the drugs (OR = 1.04 95% CI 0.49–2.23, P = 1.00), as shown in [Figure 3]. An insignificant Q statistic (P = 1.00) indicated the absence of heterogeneity (I2 = 0%). The pooled OR was calculated using the random effect model.

- Duration of oxygen administration in azithromycin group versus placebo group.
DISCUSSION
Bronchiolitis affects over 3 million children annually.[8] It is diagnosed in children below 2 years, commonly in infants below 6 months of age. Respiratory syncytial virus is the microbiological agent responsible in 50–80% of the cases.[2] Other viruses may include rhinovirus, influenza virus, parainfluenza virus, and coronavirus. It causes airway inflammation, mucus production, and obstruction in the lower respiratory tract. These effects may manifest as air trapping due to ball-valve effect, leading to hyperinflation seen in chest X-rays, wheezing, cough, or coryza. The clinical spectrum varies from mild symptoms, requiring home-based treatment, to respiratory distress requiring hospitalization such as rapid breathing or use of accessory muscles of respiration. They may present with either fever or hypothermia (in young infants). National Institute for Health and Care Excellence guidance for bronchiolitis recommends only hydration and oxygen supplementation as treatment for bronchiolitis.[10]
The American Academy of Pediatrics recommends oxygen and hydration strongly and hypertonic saline nebulization as a moderate recommendation. Other options such as corticosteroids, racemic epinephrine, antibiotics, saline nasal drops, and nasal suctioning are not recommended routinely.[11]
Despite bronchiolitis being a viral infection, antibiotics are being given frequently. This is probably done considering the possibility of secondary bacterial infection or pneumonia. The additional conundrum is due to difficulty in differentiating between bronchiolitis and pneumonia since both may present with fever, respiratory difficulty, crepitations, leukocytosis, and elevated C-reactive protein. Wheeze may be likely in viral cases. Chest X-ray may not always reveal signs of consolidation in bacterial pneumonia. These factors may be increasing the prescription of antibiotics in bronchiolitis. However, the alterations caused by antibiotics in the gut microbiome must be kept in mind, which may lead to long-term immunological or gastrointestinal consequences. The unnecessary use of antibiotics in the treatment of viral infections is a major cause of an increase in drug resistance.[12]
Azithromycin is commonly prescribed for respiratory infections due to its broad-spectrum coverage including intracellular organisms such as Mycoplasma, Chlamydia, and Moraxella species. In addition to anti-bacterial effect, it has also been found to modulate the function of neutrophils, epithelial cells, and macrophages and has potential anti-viral activity (rhinovirus).[5] Due to these additional effects, its use has been studied for bronchiolitis.
In the present study, all four RCTs [4,5,8,9] included in this meta-analysis, which showed that azithromycin for acute bronchiolitis in infants did not improve the outcomes in terms of either reduction in the length of hospital stay (MD = 1.21, 95% CI 0.53–2.78, P = 0.80), heterogeneity (I2 = 0%). There was no reduction in the duration of oxygen requirement, although there were differences in the values. In two studies, analyzing the length of hospital stay shows that there was no difference in intervention and control groups.[5,9] In the other two studies, there is reduction in length of stay in the azithromycin group but not statistically significant.[4,8] Analyzing the duration of oxygen requirement, there was no difference in both groups in a study by Pinto et al. and a statistically insignificant reduction in intervention group in study McCallum et al.[8,9] In contrast, two studies by Kneyber et al. and McCallum et al., the duration of oxygen requirement was more in the azithromycin group although not statistically significant.[4,5] This could be explained by the fact that not all studies had well-defined and uniform indications for hospitalization and duration of oxygen administration. Since the diagnosis is clinical, viral detection was not done in all studies. Azithromycin was initiated within 24 h of admission and three studies and within 48 h in other study. Furthermore, there was no consensus on dose and duration in all studies. No subgroup analysis depending on severity of bronchiolitis was done to know its usefulness in severe bronchiolitis. Other outcomes in two studies by McCallum et al. showed reduction in nasopharyngeal swab bacteriology 48 h post-azithromycin. Kneyber et al. and Pinto et al. have studied the need for adjuvant bronchodilator, corticosteroid, or antibiotic therapy but none were significant. No adverse events were reported due to azithromycin in all studies.
The strength of our study is the inclusion of good quality double-blinded RCT out of which two were multicenter trials. Despite this, our systematic review has its own limitations like inclusion of only four trials with limited sample size.
CONCLUSION
The results of our meta-analysis show that azithromycin for acute bronchiolitis does not reduce the length of hospitalization or duration of oxygen requirement. Due to low risk of secondary bacterial infections in those with bronchiolitis of <1%, routine of antibiotics to reduce the severity of illness may not be recommended. The rise of antibiotic resistance and alteration of gutmicrobiome also need to be considered while prescribing antibiotics for viral infections.
Authors’ contributions
Drs Thandapani K and Tamilarasan P had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Drs Mohan R and Mary JJF analyzed and did data interpretation. Drs Thandapani K, Tamilarasan P, and Mohan R contributed equally. Dr Kalaiselvan G was the senior for the study.
Concept and design: Thandapani K, Tamilarasan P, Acquisition of data: Thandapani K, Tamilarasan P, Mohan R, Analysis, or interpretation of data: Mohan R, Mary JJF, Drafting of the manuscript: Thandapani K, Tamilarasan P, Mohan R, Critical revision of the manuscript for important intellectual content: Kalaiselvan G, Statistical analysis: Mohan R, Mary JJF. Administrative, technical, or material support: Kalaiselvan G.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171-8.e1.
- [CrossRef] [PubMed] [Google Scholar]
- How to reduce the use of antibiotics in infant bronchiolitis? Acta Paediatr. 2020;109:1086-7.
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: A randomized equivalence trial. Pediatr Pulmonol. 2008;43:142-9.
- [CrossRef] [PubMed] [Google Scholar]
- Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: A multicentre, randomized, placebo-controlled trial. Front Pediatr. 2015;3:32.
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin administered for acute bronchiolitis may have a protective effect on subsequent wheezing. J Bras Pneumol. 2020;46:e20180376.
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin therapy during respiratory syncytial virus bronchiolitis: Upper airway microbiome alterations and subsequent recurrent wheeze. J Allergy Clin Immunol. 2016;138:1215-9.e5.
- [CrossRef] [PubMed] [Google Scholar]
- A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: A randomised, placebo-controlled trial. PLoS One. 2013;8:e74316.
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: A randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012;161:1104-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bronchiolitis in children: Diagnosis and management. 2015. London: NICE; Available from: https://www.nice.org.uk/guidance/ng9 [Last accessed on 2023 Jul 12]
- [Google Scholar]
- Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-502.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of antibiotic resistance and host-microbiome interactions in the human upper respiratory tract during influenza infection. Microbiome. 2020;8:39.
- [CrossRef] [PubMed] [Google Scholar]







