Translate this page into:
The effect of paracetamol on the parasitological diagnosis of Plasmodium falciparum malaria

-
Received: ,
Accepted: ,
How to cite this article: Magzob M, Al Sammani A, Al Siddiq A. The effect of paracetamol on the parasitological diagnosis of Plasmodium falciparum malaria. Glob J Health Sci Res 2023;1:133-41.
Abstract
Objectives:
Up until the present time, malaria represents an immense public health problem worldwide with a significant morbidity and mortality rate in many developing countries including Sudan. The use of antipyretics and analgesics in the settings of malaria has been a matter of contention over a vast period of time. Now, it is widely believed among the public and some health professionals in Sudan that the antipyretic and analgesic paracetamol disturbs the accuracy of the parasitological diagnosis of malaria. This study investigates the magnitude and effects of that belief through a descriptive study and examines the actual effect of paracetamol on the parasitological diagnosis of falciparum malaria through a clinical trial.
Material and Methods:
This is a conjoined study that includes a prospective and descriptive study and a randomized, placebo-controlled, double-blind, and parallel-group clinical trial. The descriptive study included 846 participants from various states in Sudan. The targeted study subjects were the public and health professionals and the study was conducted utilizing an online-based questionnaire. The clinical trial included 325 patients with uncomplicated falciparum attending two primary healthcare outpatient clinics in Gezira state, Sudan. The patients were randomly allocated to receive either placebo tablets or 500 mg of oral paracetamol after the first blood smear sample was taken. The second blood smear samples were taken 2 h after taking treatment. Parasite density estimation and other parasitological data were obtained from each sample before and after treatment.
Results:
The majority of the public thinks that paracetamol affects the parasitological diagnosis of malaria (76.21%, n = 330/433). Personal experience and family and friends were the most dominant information sources for the public (31.20% and 28.01% respectively), while scholarly articles were the most common source of information for the health professional group (45.03%, n = 186/413). No significant differences between the parasitological findings acquired before and after taking the treatments among both the paracetamol group (P = 0.22) and the placebo group (P = 0.12). The parasite density mean for the paracetamol group differed by 16.31 P/μL after taking paracetamol, while the placebo group parasite density mean differed by 15.34 P/μL. The treatments did not inflict impacts on the advanced microscopic features of Plasmodium falciparum blood smears.
Conclusion:
Paracetamol does not affect the parasitological diagnosis of falciparum malaria.
Keywords
Parasite density
Presenting symptoms
Prevalence
Knowledge
INTRODUCTION
Malaria remains a major cause of morbidity and death. Globally, there were an estimated 229 million malaria cases and 409000 deaths in 2019 in 87 malaria-endemic countries. The Republic of Sudan is the leading contributor to malaria in the World Health Organization (WHO) Eastern Mediterranean Region, accounting for about 46% of cases in the region. Species of Plasmodium falciparum accounted for 77.8% of all reported malaria cases.[1]
The falciparum species is the most pathogenic among the five known species of the genus Plasmodium due to its highly replicative nature and its ability to modify the cell membrane of the infected erythrocytes by manifesting parasite-derived cell surface proteins which cause sequestration of red blood cells (RBCs) and consequently avoidance of splenic parasite clearance and microvascular obstruction.[2]
The signs and symptoms of malaria are non-specific thus parasitological diagnosis is required when malaria is clinically suspected. The two methods used routinely for parasitological diagnosis of malaria are light microscopy and immunochromatographic rapid diagnostic tests (RDTs); however, light microscopy is still considered the “field standard.”[3] The WHO defines uncomplicated falciparum malaria as “A patient who presents with symptoms of malaria and a positive parasitological test (microscopy or RDT) but with no features of severe malaria.”[4] In developing countries like Sudan, Health facilities rely heavily on light microscopy in diagnosing malaria as it has a relatively low cost, high sensitivity, and specificity and allows the detection of other conditions using the same setting.
It is widely believed among the public and some health professionals in Sudan that paracetamol disturbs the diagnostic accuracy of light microscopy in detecting malaria parasites. Paracetamol (N-acetyl-p-aminophenol, also known as acetaminophen) is a synthetic non-opiate drug that produces analgesia and antipyresis and some studies have shown that it prolongs parasite clearance time in P. falciparum malaria.[5-7]
The hypothesis believed by the public and some health professionals in Sudan is that paracetamol causes plasmodium parasites to appear less in the peripheral blood because it may induce sequestration of parasite cells in deep vascular beds, thus it interferes with the detection and quantification accuracy of malaria parasites when investigated with light microscopy. This hypothesis is not evidentially supported; however, it gave rise to a popularly practiced precautionary measure that is conducted by both the public and some health professionals in Sudan, where clinically suspected malaria patients are instructed to not consume paracetamol as an analgesic or antipyretic drug before taking a blood sample for light microscopy, as it may result in diagnostic inaccuracies.
This study aims to empirically test the effect of paracetamol on the parasitological diagnosis in patients with uncomplicated falciparum malaria, to either refute the aforementioned hypothesis or to confirm its validity and promote further studies in the matter.
MATERIAL AND METHODS
Study design and settings
We conducted a prospective and descriptive study to investigate the prevalence, knowledge, and attitude of the public and health professionals concerning the effect of paracetamol on the parasitological diagnosis of malaria. Afterward, we conducted a randomized and clinical trial to investigate the actual effects of paracetamol on the parasitological diagnosis of falciparum malaria.
The descriptive study
Instrument
We utilized an online-based pre-tested 22 items questionnaire. The questionnaire was divided into five sections. The first section was concerned with the demographic data of the participant. The second section inquired about the frequency, investigations, and management received in the last falciparum malaria infection of the participant. The third section was concerned with antipyretics and analgesics knowledge and use. The fourth section explored the knowledge and practice of the hypothesis of concern (paracetamol affects the parasitological diagnosis of falciparum malaria). Finally, the fifth section was exclusive to the health professionals and inquired about the effects of the hypothesis on medical practice.
Sampling criteria, study subjects, and collection of data
The study was conducted using online-based simple random sampling and the sample size was determined by Cochran’s criteria for 95% confidence level. We published the questionnaire through invitational messages distributed by email and social media to the members of the Sudanese communities. The questionnaire distribution and data collection were executed by assigned collaborators covering all the states in Sudan. The targeted study subjects were the public and health professionals with the aim of equal participation among the two groups. The data collection period was from January 2022 to May 2022. The participants were allowed to respond in their own time and privacy for the questionnaire was online-based and participation was entirely voluntary. Anyone below the age of 18 years or unwilling to give informed consent was excluded from the study.
Analysis of data
Data were collected using Microsoft Excel software and all statistical analyses were performed using IBM SPSS statistics software, version 25. Descriptive statistics were used to summarize and illustrate the qualitative questions’ outcome variables after categorization. Bivariate Pearson correlation was used to identify and illustrate the association between the significant outcomes and different demographic variables. P < 0.05 was considered statistically significant.
The clinical trial
Study design and settings
This is a randomized, placebo-controlled, double-blind, and parallel-group clinical trial conducted in two primary healthcare facilities in Wad-Medani locality, Gezira state, central Sudan.
Sampling
We used the limit of sensitivity of microscopes for malaria detection (100 parasites/μL) as a significant difference for the study. The sample size was calculated for a significance level of 0.05 and power of 0.80 with estimating a standard deviation of 3. Therefore, more than 300 patients were required for each treatment group to detect the difference between the two groups.
Study subjects
This study included 325 patients attending two primary health-care outpatient clinics in Gezira state, Sudan, all of whom were clinically diagnosed with uncomplicated falciparum malaria and underwent confirmation by blood smear examination under light microscopy. The study was conducted between January 2022 and May 2022.
Inclusion criteria
The following criteria were included in the study:.
Age >18
Clinical and parasitological diagnosis with uncomplicated falciparum malaria
Absence of any exclusionary criterion.
Exclusion criteria
The following criteria were excluded from the study:
Patients with severe malaria
Patients with pathologic hematological conditions
Patients with concurrent parasitological infection
If the initial blood film examination is negative
Patients with HIV infection history
Patients with a history of allergic reactions to paracetamol or any derivative of acetanilide
Patients that used antipyretic or non-steroidal anti-inflammatory drugs within the past 3 days before attending the health facility
Patients that took drugs that alter gastric emptying within 3 days; because the absorption of paracetamol is dependent on gastric emptying.[8]
Instruments
Microscopes
We used OLYMPUS® (Biological Microscope CX43) to study the participants’ blood films before and after treatment allocation in accordance with the WHO guidelines and quality control for detecting, identifying, and quantifying malaria parasites using microscopy.[9] The microscopic investigations were handled by two experienced parasitology microscopists. We did not include immunochromatographic RDTs in our study because (1) they are not frequently used in the diagnostic settings of malaria in Sudan, (2) inability of distinguishing new infections from recently and effectively treated infections, (3) lot to lot variation, and (4) difference in quality of commercially available RDTs.
Treatments
We used paracetamol 500 mg oral tablets and placebo tablets.
Ethicality and safety
This study was conducted in accordance with the guidelines of the Declaration of Helsinki (2000) for ethical principles of medical research involving human subjects.[10] Each selected candidate received verbal information about the study procedures, goals, risks, and benefits. All participants that verbally agreed to be enrolled in the study gave written consent before randomization. The personnel in charge of ethical clearance from both study centers approved the study.
Participants were kept on observation for 2 h after taking treatment to ensure rapid management of any hypersensitivity reaction or significant side effects produced by paracetamol. Participants were informed to report any side effects or new symptoms occurring 4 h after taking treatment as a safety measure for the duration of action of paracetamol is 3–4 h.
Study procedures
At enrollment, all participants underwent a full clinical examination carried out by two physicians.
Treatment allocation
Randomization was conducted using a software. The participants from the two treatment groups blindly took either a paracetamol 500 mg oral tab (Group 1, n = 163) or a placebo pill (Group 1, n = 162) after the first blood smear sample was taken from them. The second blood smear samples were taken 2 h after taking treatment considering that the time to peak plasma concentration of paracetamol is 0.5–2 h, the time to peak effect is 1–3 h, and the duration of action is 3–4 h.[11] Potential adverse effects of the drugs were assessed immediately, after 12 h, and after 24 h in all participants of the two treatment groups. All samples were reviewed independently by two microscopists who had no information about the clinical characteristics of the study, patients and samples data nor the result of any other diagnostic test performed on the same sample. However, each microscopist was given a written form with the areas that the study focuses on to investigate. When the two microscopists failed to reach an agreement, a blinded third microscopist reviewed the sample.
Microscopic investigation
Microscopic procedures aimed mainly at slide reading, detection, and quantification of malaria parasites. Chromatin dots, condition of RBCs, condition of white blood cells (WBCs), Maurer’s cleft, and parasites condition were also studied in each sample both before and after the treatment. Parasite density estimation was obtained by counting parasites against WBCs on thick films (Parasite density/μL = Number of parasites counted × WBC count/μL ÷ Number of WBCs counted), while other measures were studied under thin films. Density calculations were done using a computer software assuming a WBCs value of 8000/μL.
Statistical analysis
Patients and samples data were collected using Microsoft Excel software and all statistical analyses were performed using IBM SPSS statistics software, version 25.
RESULTS
The descriptive study
Study sample
Overall 846 were enrolled to fill out the questionnaire, of which 413 were health professionals and 433 were from the general public. The participants from both groups were mostly Sudanese (98.81%, n = 836), and females were the majority (59.69%, n = 505). The age mean of the study population was 32.83. All of the participating health professionals had a university educational level with physicians being the majority of the participants. The demographic characteristics of the participants are shown in [Table 1].
| Characteristic | Study group | Study Population (n=846) |
|
|---|---|---|---|
| Health Professionals (n=413) |
The public (n=433) |
||
| Age | |||
| Mean | 37.01 | 28.65 | 32.83 |
| Range | 18–55 | 18–39 | 18–55 |
| Sex (%) | |||
| Male | 34.86 (n=144) | 45.49 (n=197) | 40.31 (n=341) |
| Female | 65.14 (n=269) | 54.51 (n=236) | 59.69 (n=505) |
| Nationality (%) | |||
| Sudanese | 98.06 (n=405) | 99.53 (n=431) | 98.81 (n=836) |
| Non-Sudanese | 1.94 (n=8) | 0.47 (n=2) | 11.82 (n=10) |
| Educational level (%) |
|||
| Elementary | Nil | 16.39 (n=71) | 8.39 (n=71) |
| Intermediate | Nil | 22.63 (n=98) | 11.58 (n=98) |
| Secondary | Nil | 36.48 (n=158) | 18.67 (n=158) |
| University | 100 (n=413) | 24.48 (n=106) | 61.34 (n=519) |
| Profession of health professionals (%) | |||
| Physician | 27.11 (n=112) | ||
| Physician assistant | 24.45 (n=101) | ||
| Laboratory technician | 21.30 (n=88) | ||
| Nurse | 17.43 (n=72) | ||
| Pharmacist | 9.68 (n=40) | ||
Malaria infection frequency, investigations and management received
Most of the participants reported having malaria infection in the past 30 days (57.91%, n = 490/846) with health professionals accounting for the majority of the reporting (52.65%, n = 258/490). Health professionals had higher infection frequency than the public (mean; 3.04 and 2.55, respectively, for the past 6 months). The vast majority of the health professionals group utilized private laboratory services to diagnose their last malaria infection (90.55%, n = 374/413) while most of the public relied on physicians/physician assistants to diagnose and confirm their malaria infection (58.89%, n = 255/433). Most of the health professionals self-treated themselves in their last malaria infection (92.73%, n = 383/413); in contrast, the vast majority of the public relied on health professionals to manage their last malaria infection (96.30%, n = 417/433). [Table 2] illustrates malaria infection frequency, investigations and management received among the two sample groups.
| Variable | Study group | Study population (n=846) |
|
|---|---|---|---|
| Health professionals (n=413) | The public (n=433) | ||
| Malaria infection in the last 30 days (%) | |||
| Yes | 62.46 (n=258) | 53.57 (n=232) | 57.91 (n=490) |
| No | 37.53 (n=155) | 46.42 (n=201) | 42.08 (n=356) |
| Number of confirmed Malaria infections in the last 6 months | |||
| Mean | 3.04 | 2.55 | 2.80 |
| Range | 1–5 | 0–6 | 0–6 |
| The source of diagnosis of the last malaria infection (%) |
|||
| Physician/Physician assistant | 7.26 (n=30) | 58.89 (n=255) | 33.68 (n=285) |
| Private laboratory | 90.55 (n=374) | 37.41 (n=162) | 63.35 (n=536) |
| Self-diagnosis | 2.17 (n=9) | 3.69 (n=16) | 2.95 (n=25) |
| Management source (%) | |||
| Self-medication | 92.73 (n=383) | 3.23 (n=14) | 46.92 (n=397) |
| Health professional | 7.26 (n=30) | 96.30 (n=417) | 52.83 (n=447) |
| Traditional medicine | Nil | 0.04 (n=2) | 0.02 (n=2) |
The use of paracetamol before the parasitological diagnosis of malaria
When asked about the use of paracetamol before the parasitological diagnosis of the last malaria infection, only 24% of the public reported that they did take paracetamol before the laboratory investigation compared to 64% of health professionals who reported taking paracetamol. [Figure 1] illustrates the variance between the two sample groups in regard to taking paracetamol before the parasitological diagnosis.
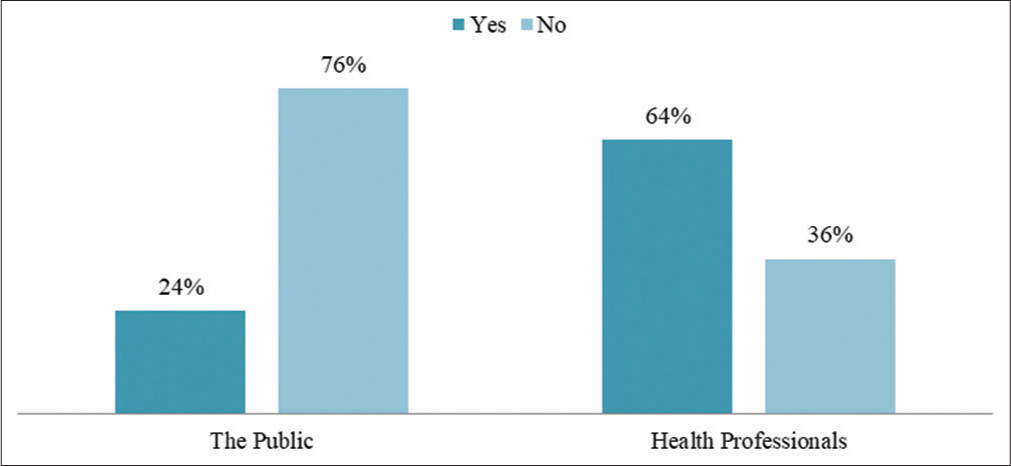
- The use of paracetamol before diagnosis confirmation in the last malaria infection.
The prevalence of the phenomenon
When asked if paracetamol affects the parasitological diagnosis of malaria, the majority of the participants from the public answered yes (76.21%, n = 330/433) while only (4.60%, n = 19/413) of the health professionals answered yes. The dominant information sources varied among the two study groups where personal experience and family and friends were the most dominant information sources for the public (31.20% and 28.01%, respectively), while scholarly articles were the most common source of information for the health professional group (45.03%, n = 186/413). The details of the responses concerning this topic are shown in [Table 3].
| Knowledge variable | Study group | Study population (n=846) |
|
|---|---|---|---|
| Health professionals (n=413) | The public (n=433) | ||
| Paracetamol affects the parasitological diagnosis of malaria (%) |
|||
| Yes | 4.60 (n=19) | 76.21 (n=330) | 41.25 (n=349) |
| No | 78.93 (n=326) | 6.69 (n=29) | 41.96 (n=355) |
| I do not know | 7.74 (n=32) | 17.09 (n=74) | 12.52 (n=106) |
| Information source (%) | |||
| Family and friends | 0.48 (n=2) | 54.27 (n=235) | 28.01 (n=237) |
| The internet | Nil | 3.46 (n=15) | 1.77 (n=15) |
| Personal experience | 19.61 (n=81) | 42.26 (n=183) | 31.20 (n=264) |
| Work experience | 34.86 (n=144) | Nil | 17.02 (n=144) |
| Scholarly article | 45.03 (n=186) | Nil | 21.98 (n=186) |
Notable correlations
The majority of the participants from the public who think that paracetamol affects the parasitological diagnosis of malaria were females (54.84%, n = 181/330), Sudanese (100%, n = 330/330), with an age mean of (29.22) secondary education was the most common highest education level among them (36.66%, n = 121).
The clinical trial
Study sample
Patients with pre-confirmed diagnosis of uncomplicated falciparum malaria were enrolled when eligible at the two study centers (n = 325). A total of 650 blood smear samples were collected and examined during the study period. The demographic characteristics and vital signs of the participants are shown in [Table 4].
| Characteristic (n=325) | Treatment group | -value | |
|---|---|---|---|
| Paracetamol (n=163) | Placebo (n=162) | ||
| Age | 0.85 | ||
| Mean | 34.72 | 39.63 | |
| Range | 18–52 | 20–67 | |
| Sex, M/F | 70/93 | 62/101 | 0.39 |
| Residence | 0.58 | ||
| Gezira state | 152 | 158 | |
| Elsewhere | 11 | 5 | |
| Temperature | 0.40 | ||
| Mean | 38.5 | 38.1 | |
| Range | 37.2–39.2 | 37.5–39.0 | |
| Pulse rate (P/min) | 0.43 | ||
| Mean | 104 | 98 | |
| Range | 72–150 | 64–126 | |
| Respiratory rate | 0.59 | ||
| Mean | 20 | 20 | |
| Range | 14–26 | 12–22 | |
| Blood pressure (SBP/DBP) | 0.71 | ||
| Mean | 132.23/84.78 | 136.36/88.92 | |
| SD | 15.04/10.52 | 11.23/9.07 | |
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, SD: Standard deviation
Presenting symptoms of the patients
All of the participating patients underwent full clinical assessment before enrollment and randomization to isolate eligible patients. After enrollment, patients underwent a second clinical assessment for the study, which revealed that headache is the most common main complaint among patients with uncomplicated falciparum malaria followed by fever and chills as shown in [Table 5].
| Symptom | Treatment group | P-value | |
|---|---|---|---|
| Paracetamol (n=163) (%) |
Placebo (n=162) (%) | ||
| Headache | 63.19 (n=103) | 69.13 (n=112) | 0.31 |
| Fever, chills, sweating | 58.89 (n=96) | 25.92 (n=42) | 0.19 |
| Myalgia | 26.99 (n=44) | 31.14 (n=51) | 0.31 |
| Joint pain | 20.85 (n=34) | 19.13 (n=31) | 0.15 |
| Nausea, vomiting | 7.36 (n=12) | 4.93 (n=8) | 0.16 |
| Asymptomatic | 2.45 (n=4) | 1.23 (n=2) | 0.13 |
| Other | 0.61 (n=1) | 1.23 (n=2) | 0.15 |
Parasitological findings
The parasite density readings and parasite microscopic features were statistically close and had no significant difference among the two treatment groups before treatment allocation as shown in [Table 6]. The mean parasite density for the whole sample was 2485.48 P/μL (95% CI of the whole mean = 2485.48 ± 412 P/μL). The majority of the parasite density readings were less than 1000 P/μL (56.61% of the whole sample) among the two treatment groups with the ring stage being the dominant presenting stage.
| Parasitological data | Treatment group | |
|---|---|---|
| Paracetamol (n=163) |
Placebo (n=162) | |
| Parasite density (Per μL) | ||
| Low (<1000) | 58.89% (n=96) | 54.32% (n=88) |
| Moderate (1000–4,999) | 33.12% (n=54) | 36.41% (n=59) |
| High (5000–99,999) | 7.97% (n=13) | 9.87% (n=16) |
| Mean | 2361.96 P/μL | 2609.01 P/μL |
| 95% CI of the mean | 2361.96±391 P/μL | 2609.01±433 P/μL |
| Geometric mean | 1936.52 P/μL | 2052.23 P/μL |
| Range | 200–10,640 P/μL | 530–11,252 P/μL |
| SD | 2548.94 | 2822.61 |
| Parasite stage | ||
| Ring | 96.93% (n=158) | 99.38% (n=160) |
| Schizont | 14.72% (n=24) | 5.55% (n=9) |
| Gametocytaemia | 2% (n=5) | 4.32% (n=7) |
CI: Confidence interval, SD: Standard deviation
There were no significant differences between the parasitological findings acquired before and after taking the treatments among both the paracetamol group (P = 0.22) and the placebo group (P = 0.12). The parasite density readings after taking treatments remained within the confidence interval range of the readings before taking the treatments for both treatment groups as shown in [Table 7]. The clustering in parasite density parameters remained the same with an insignificant difference in the mean among the two treatment groups. The parasite density mean for the paracetamol group differed by 16.31 P/μL after taking paracetamol, while the placebo group parasite density mean differed by 15.34 P/μL. A comparison between the parasite density readings for both groups before and after taking the treatments is illustrated in [Figure 2]. More schizonts were detected in the paracetamol group after taking the treatment; however, the proportions of the detected parasite stages remained unaffected. All of the patients continued to have falciparum malaria parasites in their blood smear regardless of the treatment taken.
| Parasitological data | Treatment group | |
|---|---|---|
| Paracetamol (n=163) |
Placebo (n=162) |
|
| Parasite density (Per μL) | ||
| Low (<1000) | 58.89% (n=96) | 54.32% (n=88) |
| Moderate (1000–4,999) | 33.12% (n=54) | 36.41% (n=59) |
| High (5000–99,999) | 7.97% (n=13) | 9.87% (n=16) |
| Mean | 2378.27 P/μL | 2593.67 P/μL |
| 95% CI of the mean | 2378.27±388 P/μL | 2593.67±424 P/μL |
| Geometric mean | 1951.22 P/μL | 2050.41 P/μL |
| Range | 280–10,430 P/μL | 520–11,338 P/μL |
| SD | 2598.05 | 2766.11 |
| Parasite stage | ||
| Ring | 96.93% (n=158) | 99.38% (n=160) |
| Schizont | 15.95% (n=26) | 5.55% (n=9) |
| Gametocytaemia | 2% (n=5) | 4.32% (n=7) |
CI: Confidence interval, SD: Standard deviation
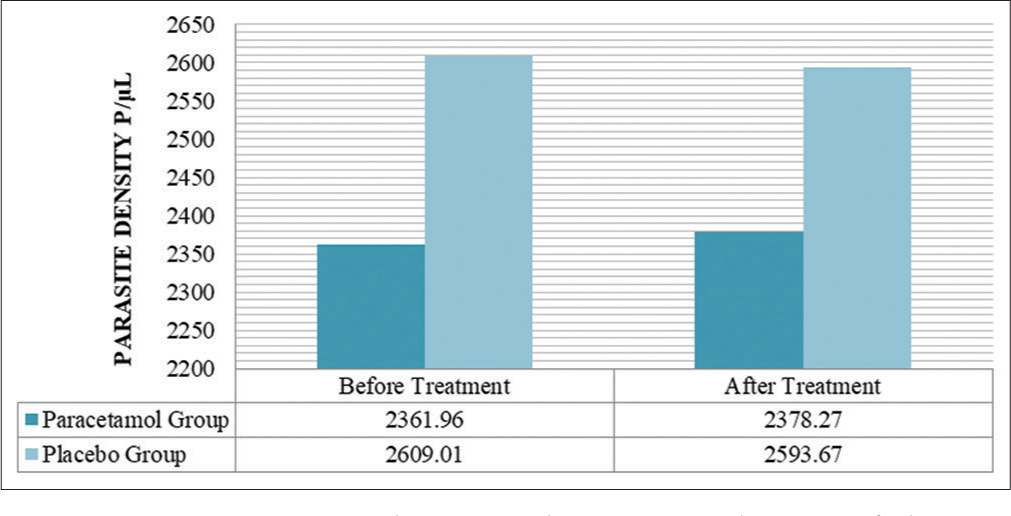
- A comparison between the parasite density of the two treatment groups before and after taking treatments.
The impact of treatments on the advanced microscopic features of P. falciparum blood smears
The appearance of the infected erythrocytes was reported to be normal in most of the blood smears before and after taking treatment. Multiple infections of erythrocytes were common with a maximum of two chromatin dots for a single erythrocyte in both treatment groups before and after treatment. No significant variation was appreciated in the number of infections or chromatin dots after taking the treatments. Twelve samples presented erythrocyte appearance distortion and all of which were carrying gametocytes which is a common phenomenon. The condition of leukocytes and parasites remained unchanged throughout the study for both treatment groups. Maurer’s cleft was reported in 12 blood smears both before and after treatment and all of which were detected with a schizont stage.
The impact of the treatments
The majority of the paracetamol group patients who complained of headache and fever reported a significant decrease in symptom severity after taking the treatment. About 88.34% reported headache relative subsidence while 76.04% reported relative subsidence of fever. Only 18% of the placebo group patients who had headaches reported a significant decrease in headache severity and none reported a relative subsidence of fever all of which is shown in [Figures 3 and 4]. Myalgia and joint pain were reported equally before and after treatment for both treatment groups.
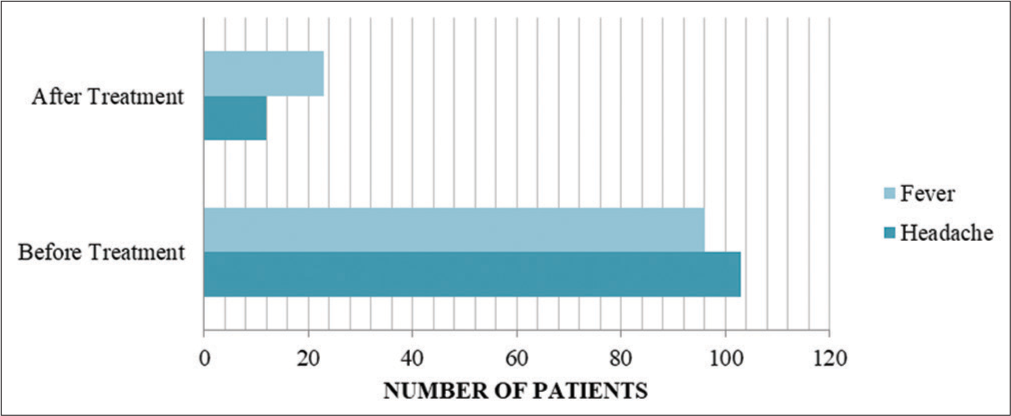
- A comparison between the presence of headache and fever before and after taking treatment among the paracetamol group patients.
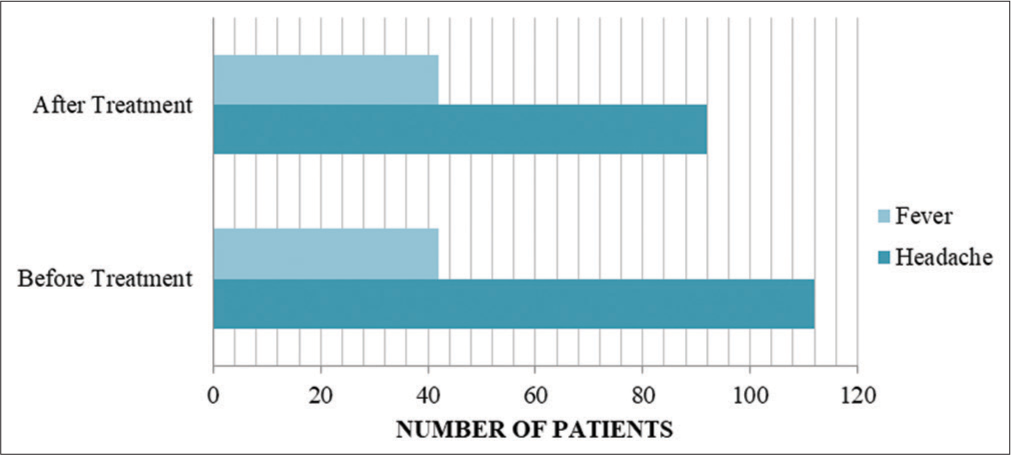
- A comparison between the presence of headache and fever before and after taking treatment among the placebo group patients.
DISCUSSION
The WHO recommends the use of antipyretics in the setting of uncomplicated falciparum malaria when the core temperature is >38.5°C. Furthermore, the WHO recommends paracetamol as an antipyretic for uncomplicated falciparum malaria at a dose of 15 mg/kg, affirming that it is safe and well tolerated.[4] Despite the WHO recommendations, a significant number of the public in Sudan still believe that the ingestion of paracetamol might hinder the accuracy of the parasitological diagnosis of uncomplicated falciparum malaria as shown in the results of our study. This widespread pseudoscientific belief led to an unnecessary practice by both the public and some health professionals where patients are forbidden to use paracetamol as an antipyretic or analgesic before the parasitological diagnosis of malaria is established.
Paracetamol is one of the most widely used over-the-counter medicines and its efficacy is well-studied and proven especially in managing mild-to-moderate pain and fever, which are among the most common presenting symptoms of malaria.[12] Our study revealed headache and fever were the most common presenting symptoms among patients with uncomplicated falciparum malaria (66.16% and 42.40%, respectively), which conveys the main reasons patients seek paracetamol at the beginning of symptoms. A study conducted by Aguado et al. concerning the antipyretic effectiveness of paracetamol revealed that paracetamol can achieve a maximum temperature drop of −1.09 ± 0.77 which is a significant decrease.[13] The faulty prohibition of access to paracetamol where it is evidentially recommended would only worsen the patient’s experience of the disease; especially in the case of falciparum malaria for it is an endemic disease in Sudan with a significant reinfection rate with fever and headache as the common presenting symptoms as shown in our study and several others.[14,15]
A study conducted in Gabon which involved 50 children with falciparum malaria that were treated with intravenous quinine and received either mechanical antipyresis alone, or in combination with paracetamol revealed that parasite clearance time was significantly prolonged in patients who received paracetamol with a time difference of 16 h. In the previous study, the children treated with paracetamol had a lower induced concentration of tumor necrosis factor (TNF) and reduced production of oxygen radicals.[6] This could be explained by the antiparasitic effects of fever and TNF.[16,17] An age-structured coupled Markov chain model was developed by Gravenor and Kwiatkowski to examine the temperature effects of fever on the intra-host population dynamics of P. falciparum disclosed that “during the primary infection of a non-immune host, a typical episode of fever can affect density-dependent regulation of the parasite population, maintaining cycles of parasitemia and promoting synchronous parasite growth.”[18] However, when Hugosson et al. compared the combination of sulfadoxine/ pyrimethamine (SP) plus chloroquine, chloroquine alone, SP alone, and SP plus paracetamol in the treatment of uncomplicated P. falciparum malaria in Tanzania, they found that the addition of chloroquine or paracetamol to SP improved the clinical outcome, but did not affect the parasitologic response or antibody production.[19]
Conversely, some studies scrutinized the effects of malaria infection on the pharmacokinetics and pharmacodynamics of paracetamol. A study conducted on rats infected with Plasmodium berghei, a rodent malarial model, to examine the effect of malaria infection on paracetamol disposition, found that malaria infection causes a significant decrease in clearance time and a significant prolongation of the elimination half-life of paracetamol.[20] On the other hand, a study conducted with Thai patients measuring plasma concentrations and the urinary excretion of paracetamol and its Phase II metabolites during and after treatment of falciparum malaria found that the apparent oral clearance, the elimination half-life of paracetamol and apparent volume of distribution of paracetamol were similar during malaria and convalescence.[21]
Despite the controversies surrounding the use of paracetamol in the management of malaria, paracetamol is undoubtedly a renowned symptomatic treatment for pain and fever. We conducted this study with the sole purpose of enlightening the public and health professionals about the importance of evidence-based medicine and the hazardous consequences of practicing upon a pseudoscientific basis. Although the resources on the topic are scarce and not well documented, the belief of paracetamol’s effect on the parasitological diagnosis of malaria is notably prevalent among the people of Sudan. According to our best knowledge, there are no published works in Sudan that describe the prevalence of such knowledge and practice, thus we were compelled to instigate this investigation.
CONCLUSION
Despite the wide spread belief that paracetamol disturbs the detection and quantification process of malaria parasites among the public, we found that paracetamol does not affect the parasitological diagnosis of falciparum malaria.
Acknowledgments
We would like to express our deep thanks and gratitude to AlWaha and Arkaweet health care centers for providing their facilities to conduct this study. The completion of this study could not have been accomplished without the support of our colleague Katsuragi Kyoko for her generous support and Mr. Al Sammani for his notable contribution in the rhetoric and composition aspects of this manuscript. We would also like to thank all of the participants that took part in both the questionnaire and the clinical trial.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Malaria pathogenesis. Cold Spring Harb Perspect Med. 2018;8:a025569.
- [CrossRef] [PubMed] [Google Scholar]
- Paracetamol: Mechanisms and updates. Contin Educ Anaesth Crit Care Pain. 2014;14:153-8.
- [CrossRef] [Google Scholar]
- Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence on the use of paracetamol in febrile children. Bull World Health Organ. 2003;81:367-32.
- [Google Scholar]
- Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings (Version 1.0) Procedure: Methods Manual. Geneva: World Health Organization; Available from: https://apps.who.int/iris/handle/10665/163782 [Last accessed on 2022 Jan 04]
- [Google Scholar]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2000;284:3043-5.
- [CrossRef] [Google Scholar]
- Clinical pharmacokinetics of paracetamol. Clin Pharm. 1982;7:93-107.
- [CrossRef] [PubMed] [Google Scholar]
- Antipyretic effectiveness of ibuprofen and paracetamol. Anal Pediatr (Barc). 2005;62:117-22.
- [CrossRef] [PubMed] [Google Scholar]
- Self-reported fever, treatment actions and malaria infection prevalence in the northern states of Sudan. Malaria J. 2011;10:128.
- [CrossRef] [PubMed] [Google Scholar]
- Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987;67:1-4.
- [Google Scholar]
- Tumor necrosis factor and severe malaria. J Infect Dis. 1991;163:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- An analysis of the temperature effects of fever on the intra-host population dynamics of Plasmodium falciparum. Parasitology. 1998;117:97-105.
- [CrossRef] [PubMed] [Google Scholar]
- Antipyretic, parasitologic, and immunologic effects of combining sulfadoxine/pyrimethamine with chloroquine or paracetamol for treating uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:366-71.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of malaria infection on paracetamol disposition in the rat. Biochem Pharmacol. 1991;41:1707-11.
- [CrossRef] [Google Scholar]
- Paracetamol disposition in Thai patients during and after treatment of falciparum malaria. Eur J Clin Pharmacol. 1995;48:65-9.
- [CrossRef] [PubMed] [Google Scholar]







