Translate this page into:
Efficacy and safety of Ibalizumab for the treatment of HIV-1 infected patients: A systematic review

*Corresponding author: Vijayasankar Palaniappan, Department of Dermatology Venereology and Leprosy, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India vijayasankarpalaniappan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Baskar Murthy A, Palaniappan V, Karthikeyan K, Mohan R, Mary JJF. Efficacy and safety of Ibalizumab for the treatment of HIV-1 infected patients: A systematic review. Glob J Health Sci Res. 2024;2:63-9. doi: 10.25259/GJHSR_23_2024
Abstract
Objectives:
Human immunodeficiency virus (HIV) infection is a significant global health concern, due to the emerging complexity in the management of infection. The emergence of novel therapeutic agents, such as ibalizumab, has provided a ray of hope for individuals living with HIV. This systematic review provides a comprehensive analysis of the efficacy and safety of ibalizumab for the treatment of HIV-1-infected patients.
Material and Methods:
Using online medical literature databases and the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines, four of the 86 articles met the acceptance criteria to be analyzed. Details such as author name, year of publication, demographic characteristics, mode, and dose of drug administration, duration of treatment, comparator if any, baseline CD4 counts, and viral load, change in CD4 count and viral load, and the adverse events were noted in the studies.
Results:
The total number of patients enrolled in each study ranged from 22 to 82 with a median age ranging from 39 to 53. Except for the open-label dose-ranging cohort study, baseline CD4 counts and post-intervention CD4 counts were assessed in all the studies. The patients who received a higher dose of ibalizumab showed an early significant rise in CD4+T-cell count at week 16 and week 48. Although the viral reduction after ibalizumab injection increases from the dose of 3 mg/kg, it was noted that beyond 10mg/kg the viral load reduction was not increasing proportionately with 25 mg/kg. The adverse effects encountered among the four studies ranged from 45% to 91%. The commonly observed adverse effects were headache, diarrhea, nausea, fatigue, somnolence, and rash.
Conclusion:
Ibalizumab demonstrates promise as a therapeutic option for individuals with multidrug-resistant HIV-1. Its unique mechanism of action and positive impact on viral load reduction and CD4 cell counts make it a valuable addition to the armamentarium of HIV treatment options.
Keywords
Ibalizumab
HIV-1
AIDS
INTRODUCTION
Human immunodeficiency virus (HIV) infection remains a significant global health concern, affecting more than 70 million individuals worldwide and approximately 35 million deaths.[1] Despite advances in antiretroviral therapy (ART), some patients experience treatment challenges due to multidrug resistance, limited treatment options, and intolerance to traditional HIV medications. The emergence of novel therapeutic agents, such as ibalizumab, has provided a ray of hope for individuals living with HIV who have exhausted conventional treatment options.[1]
Ibalizumab, a humanized monoclonal antibody, represents a groundbreaking addition to the armamentarium of HIV therapeutics. It targets the CD4 receptor, a critical component of the viral entry process, thus inhibiting viral replication. Unlike traditional ART, which primarily focuses on viral enzymes, ibalizumab offers a unique mechanism of action by directly interfering with the interaction between the virus and the host cell.[2]
The objective of this systematic review is to comprehensively evaluate the available evidence on the efficacy and safety profile of ibalizumab in the treatment of HIV. By synthesizing the existing literature, we aim to provide clinicians, researchers, and policy-makers with a comprehensive overview of the current state of knowledge regarding ibalizumab’s effectiveness, tolerability, and potential role in the management of HIV-infected patients.
MATERIAL AND METHODS
Study sources and search strategy
The present study was carried out according to PRISMA guidelines. Electronic searches were performed using PubMed, and Google Scholar for articles published on ibalizumab for HIV-1 infected individuals from their dates of inception to July 2023. To achieve the maximum sensitivity of the search strategy, the following search terms were used: “Ibalizumab,” or “TNX-355,” combined with “HIV-1,” or “Human immunodeficiency virus” or “acquired immunodeficiency syndrome” or “AIDS.” The reference lists of all the recovered articles were reviewed for further identification of relevant studies and evaluated using the inclusion and exclusion criteria. The search results were uploaded into the online systematic review program Rayyan to conduct the study selection.
Selection criteria
Eligible studies for the present systematic review included those in which randomized controlled trials (RCT) and open-label trials including dose-ranging studies in which HIV-1 infected patients were treated with Ibalizumab. All cross-sectional studies, case-control studies, reviews, case series, case reports, studies on HIV-2 infection, and studies assessing other anti-retroviral drugs were excluded from the study. Abstracts, editorials, conference presentations, reviews, and expert opinions were also excluded from the study. The search strategy employed is depicted in Figure 1.
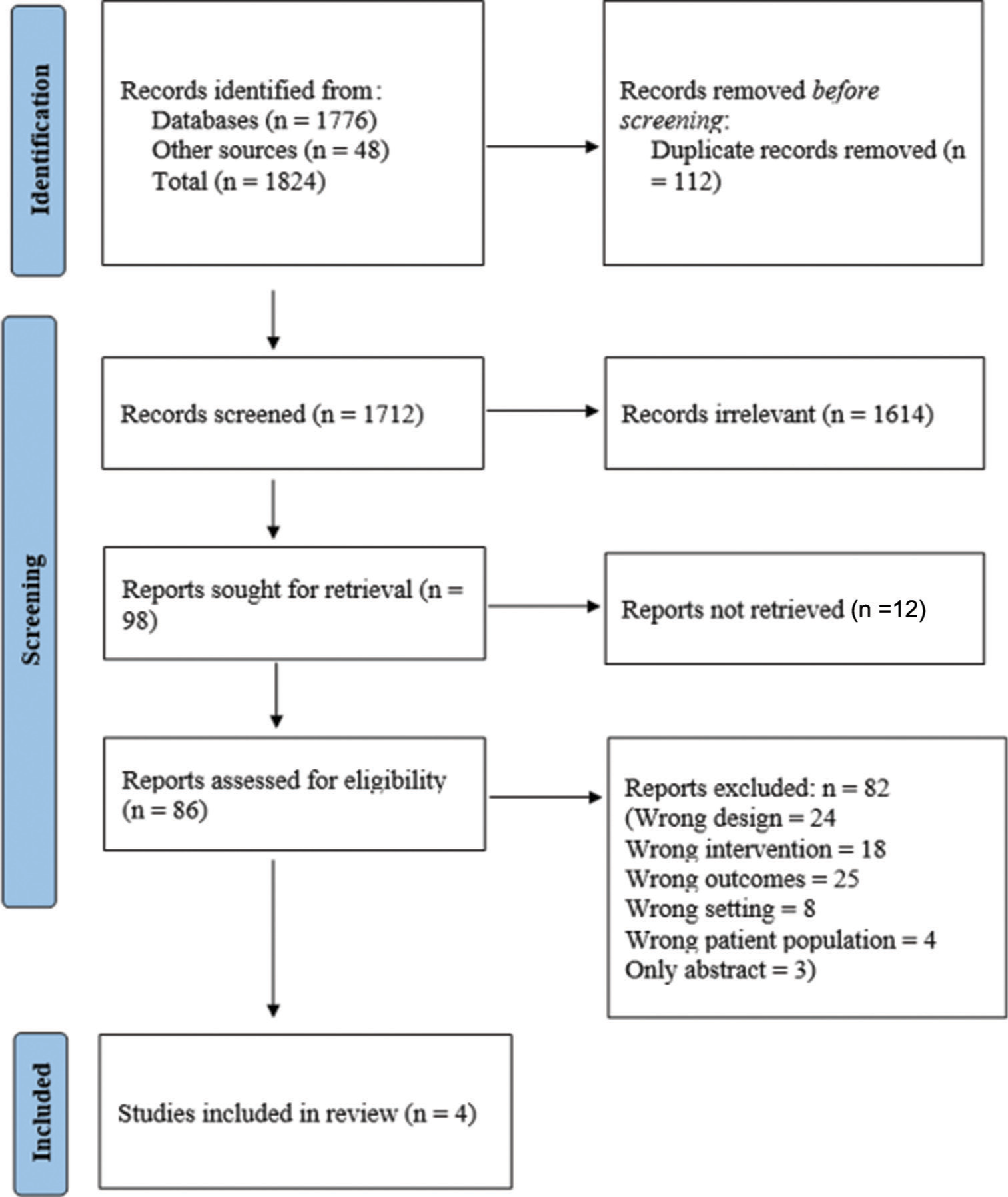
- The PRISMA flow chart for the study selection process. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Data extraction and outcome assessment
The relevant study characteristics for the review were extracted by the first and coauthor independently, related to the outcome measures from the included studies. Data extraction was guided by a predetermined checklist with author name, year of publication, age, gender, the total number of patients, mode, and dose of drug administration, duration of treatment, comparator if any, baseline CD4 counts, and viral load, change in CD4 count and viral load, and the adverse events noted in the studies. All data were extracted from article text, figures, and tables. Two investigators independently reviewed each retrieved article (ABM and VP). Discrepancies between the two reviewers were sorted out by discussion and consensus. The efficacy of ibalizumab was assessed based on the decrease in the plasma HIV -1 ribonucleic acid (RNA) levels (log10 copies/mL), and the increase in CD4 count (cells/mm3) after the initiation of ibalizumab. The safety profile was assessed based on the adverse events reported in the studies.
Quality assessment
The quality of evidence of each study was assessed using a revised Cochrane risk-of-bias tool for randomized trials (RoB 2).
RESULTS
Baseline characteristics
A total of 1824 articles were identified through two electronic database searches and from other sources such as reference lists. After removing the duplicate (112) and irrelevant (1614) records, 98 articles were sought for retrieval. Around 12 articles could not be retrieved, so we requested the authors for the full text. A total of 86 articles were assessed for eligibility and finally, four articles were included in the review. It included one randomized-controlled trial, two open-label trials, and one dose-ranging cohort study. The study was blinded in one study. On performing the risk of bias assessment by the Cochrane RoB2 tool, one study had a low risk of bias, two studies had a moderate risk of bias, and one had a high risk of bias.
Total number of patients enrolled in each study ranged from 22 to 82. The number of patients in a single-arm ranged from 3 to 40. The median age of the patients ranged from 39 to 53. The proportion of men ranged from 82% to 90%. The proportion of African descendent and European descendent population ranged from 46.33% to 80% and 6.7% to 33%, respectively. The summary of characteristics of the studies included in this systematic review is explained in Table 1. In all the studies included in the review, ibalizumab was administered intravenously. In the open-label dose-ranging cohort study, ibalizumab was given as a single infusion on Day 0 with varying doses across the five cohort arms, and the subjects were assessed over 90 days.[3] In the other studies, the treatment duration ranged from 16 weeks to 48 weeks.[4-6]
| Author | Year | Study design | Number of patients in the study | Age (range) | Ibalizumab administration method (dosage) | Duration of treatment | Comparator |
|---|---|---|---|---|---|---|---|
| Gathe et al.[4] | 2021 | Randomized clinical trial | Arm A: 28 patients Arm B: 27 patients | Arm A: 44 (28–59) Arm B: 46 (18–75) | Arm A: Alternating weekly 15 mg/kg IV infusions and placebo-9 doses, followed by 15 mg/kg infusions q2wk Arm B: Weekly 10 mg/kg IV infusions-9 doses, followed by 10 mg/kg infusions q2wk | 16–48 weeks | Placebo (n=27)-weekly placebo IV infusion for 9 doses, followed by infusions q2wk |
| Emu et al.[6] | 2018 | Open-label trial | 40 | 53 (23–65) | Control period: Days 0–6-No ibalizumab Functional monotherapy period: Days 7–13–IV Ibalizumab 2000 mg bolus Maintenance period: Day 14 to week 25-IV Ibalizumab 800 mg every 2 weeks from Day 21 | 25 weeks | N/A |
| Jacobson et al.[5] | 2008 | Open-label trial | Arm A: 9 patients Arm B: 10 patients Arm C: 3 patients | Arm A: 39 (24–60) Arm B: 42 (22–52) Arm C: 41 (36–43) | Arm A: 10 mg/kg Ibalizumab IV every week for 10 doses Arm B: Single loading dose of 10 mg/kg Ibalizumab on Day 1 followed by 5 maintenance doses of 6 mg/kg every 2 weeks Arm C: 25 mg/kg Ibalizumab every 2 weeks for 5 doses | Arm A: 16 weeksArm B: 16 weeksArm C: 32 weeks | N/A |
| Kuritzkes et al.[3] | 2004 | Open-label dose- ranging study | 30 (5 sequential cohorts of 6 each) | 42.3 (28–57) | Cohort 1: 0.3 mg/kg ibalizumab Cohort 2: 1 mg/kg ibalizumab Cohort 3: 3 mg/kg ibalizumab Cohort 4: 10 mg/kg ibalizumab Cohort 5: 25 mg/kg ibalizumab | 90 days | N/A |
HIV-1: Human Immunodeficiency Virus -1, N/A: Not allowed
Efficacy of ibalizumab in HIV-1-infected individuals
Except for the open-label dose-ranging cohort study, baseline CD4 counts and post-intervention CD4 counts were assessed in all the studies. In the double-blinded randomised control trial (RCT), the CD4 counts were assessed at week 16, week 24, and week 48. The increase in the CD4 cell count was statistically significant in arm A (15 mg/kg) at week 16 (+86 cells/µL; P = 0.05) and week 48 (+50 cells/µL; P = 0.020), and in arm B (10 mg/kg) at week 48 (+48 cells/µL; P = 0.028).[4]
In the single-group open-label trial comparing the drug efficacy in 40 patients during three periods (control period, functional monotherapy period, and maintenance period), the mean CD4 count increased from 150 cells/µL at baseline to 240 cells/µL at week 25 (mean increase of 62 cells/µL).[6]
In the open-label multidose study, patients in all three treatment arms (weekly 10 mg/kg, 10 mg/kg followed by 6 mg/kg maintenance doses, and 25 mg/kg) showed a mean increase in CD4 count from baseline with the largest increase occurring in week 1 (arms A and B) or week 2 (arm C). The mean increase in CD4 count was 238 cells/mm3, 186 cells/mm3, and 129 cells/mm3 for arms A, B, and C, respectively.[5]
In the open-label dose-ranging study, the median CD4 count was found to be 282 (165–902), but the mean or median increase in the CD4 count was not assessed in any group.[3]
The viral load after the intervention in the double-blinded RCT showed a decrease in 1.33 log10, 1.19 log10 decrease, and 0.77 log10 decrease of viral copies in the arm receiving Ibalizumab 10 mg/kg, at weeks 16, 24, and 48 compared to 15 mg/kg (1.07 log10, 0.77 log10 decrease, and 0.54 log10 decrease) and placebo (0.26 log10 decrease, 0.32 log10 decrease, and 0.22 log10 decrease).[4] In an open-label trial, the mean change in viral load from baseline was a 1.1 log10 decrease during the functional monotherapy period (days 7 to 13) and a 1.6 log10 decrease at week 25 compared to a 0.0 log10 decrease during the control period.[6] In another open-label multidose study, the maximum decrease in viral load was observed for arm A (weekly 10 mg/kg of ibalizumab) and arm C (25 mg/kg of ibalizumab every 2 weeks) of 0.95 log10 copies/mL decrease and 0.96 log10 copies/mL decrease at week 1 compared to arm B (10 mg/kg followed by 6 mg/kg every 2 weeks) with 0.83 log10 copies/mL decrease at week 2.[5] In the open-label, dose-ranging study assessing the efficacy of ibalizumab in doses 0.3–25 mg/kg, it was observed that a significant reduction of HIV-1 RNA occurred only at doses 3 mg/kg (0.56 log10 copies/mL decrease at week 1), 10 mg/kg (1.33 log10 copies/mL decrease at week 2), and 25 mg/kg (1.11 log10 copies/mL decrease at week 3).[3] The summary of the results of studies done on ibalizumab for HIV-1-infected individuals is mentioned in Table 2.
| Author | Baseline CD4 count | Change in CD4 count | Baseline viral load | Change in viral load | Adverse events (n) |
|---|---|---|---|---|---|
| Gathe et al.[4] | Arm A: 178 (38–532) Arm B: 263 (47–721) Placebo: 241 (48–715) | Week 16Arm A: +86/µL Arm B: +38/µL Placebo: +14/µL Week 24 Arm A: +51/µL Arm B: +09/µL Placebo: +5/µL Week 48 Arm A: +50/µL Arm B: +48/µL Placebo: +0/µL | Arm A: 5.2 (4.2–5.9) Arm B: 4.8 (4–5.5) Placebo: 4.8 (3.9–5.8) | Week 16 Arm A: 1.07 log10 decrease Arm B: 1.33 log10 decrease Placebo: 0.26 log10 decrease Week 24 Arm A: 0.77 log10 decrease Arm B: 1.19 log10 decrease Placebo: 0.32 log10 decrease Week 48 Arm A: 0.54 log10 decrease Arm B: 0.77 log10 decrease Placebo: 0.22 log10 decrease | Arm A: 26 Arm B: 24 Placebo: 24 |
| Emu et al.[6] | 73 (0–676)-median 150±182-mean | +62 cells/µL at week 25 (mean) | 4.6 (2.5–5.9)- median 4.5±0.8-mean | Control period Mean reduction: 0.0±0.2 Functional monotherapy period mean reduction: 1.1 log10 copies/Ml Median reduction: 1.1 log10 copies/mL Maintenance period: mean reduction: 1.6 log10 copies/mL Median reduction: 1.8 log10 copies/mL | 32 |
| Jacobson et al.[5] | Arm A: 294 (89–462) Arm B: 327 (109–458) Arm C: 338 (274–417) | Arm A: + 238 cells/µL at week 1 Arm B: + 186 cells/µL At week 1 Arm C: + 129 cells/µL at week 2 | Arm A: 4.7 (3.8–5.7) Arm B: 4.7 (3.8–5.6) Arm C: 4.9 (4.2–5.7) | Arm A: 0.95 log10 copies/mL decrease at week 1 Arm B: 0.83 log10 copies/mL decrease at week 2 Arm C: 0.96 log10 copies/mL decrease at week 1 | 10 |
| Kuritzkes et al.[3] | 282 (165–902)-median | Not assessed | 4.72 (3.57–5.67) | Cohort 1: Substantial reduction not noted Cohort 2: Substantial reduction not noted Cohort 3: 0.56 log10 copies/mL decrease at week 1 Cohort 4: 1.33 log10 copies/mL decrease at week 2 Cohort 5: 1.11 log10 copies/mL decrease at week 3 | 20 |
HIV-1: Human Immunodeficiency Virus -1
Safety
The adverse effects encountered among the four studies ranged from 45% to 91%. In the double-blinded RCT, the incidence, frequency, and intensity of treatment-emergent adverse events (TEAEs) were identical in the ibalizumab arms and placebo arms. The most frequently encountered TEAEs (≥ 10) in two ibalizumab arms and placebo were headache (17.9% and 22.2% vs. 18.5% for placebo), diarrhea (7.4% and 17.8% vs. 7.4% for placebo), nausea (3.7% and 10.7% vs. 3.7% for placebo), fatigue (10.7% and 11.1% vs 22.2% for placebo), somnolence (0 and 14.3% vs. 0% for placebo), and rash (10.7% and 14.8% vs. 0% for placebo). Sixteen serious adverse events were reported in 11 subjects including one mortality. However, none of them were deemed by investigators to be ibalizumab-related.[4]
In phase 3, open-label trial, 87% of adverse events (AEs) were mild-to-moderate in severity that included diarrhea (20%), nausea, fatigue, fever, skin rash, and dizziness (in 13% each). Serious adverse effects were encountered in 23% of the patients resulting in discontinuation of ibalizumab treatment in 13% of patients. Four deaths in the study period were not considered to be drug-related.[6] In phase 1, open-label, multidose study, the most common AEs observed were headache (27.2%), nausea, and productive cough in 13.6% of patients. Four serious AEs encountered in the study were not drug-related.[5] In the open-label, dose-ranging cohort study, 66.7% of subjects experienced at least one AE after receiving the ibalizumab. The most common AEs noted were headache (13.3%), skin rash (10%), itching, urticaria, and nasal congestion in 6.7% each.[3]
DISCUSSION
HIV infection and AIDS have posed serious public health concerns in the past few decades. The prevalence of transmitted and acquired HIV drug resistance in individuals receiving ART has significantly increased in recent years.[7-9] The emerging drug-resistant HIV-1 infection has become an impediment to ending the HIV-1 epidemic by the year 2030.[10] Although antiretroviral drugs have significantly improved the prognosis of HIV patients, the frequent side effects and resistance have led to a constant lacunae in HIV therapy. Hence, the monoclonal antibodies have shown their emergence due to targeted therapy, with minimal chances of resistance, and fewer adverse events. Ibalizumab is one such first Food and Drug Administration (FDA)-approved long-acting monoclonal antibody for HIV-1 treatment targeting CD4, thereby blocking HIV-1 entry into cells with only a few trials conducted on the efficacy and safety of the drug.
Among the four studies included in the systematic review, only one was a double-blinded RCT, while two studies were open-label trials and one was a dose-ranging study. Ibalizumab was given intravenously in all the studies. Three studies compared the various doses of ibalizumab with each other or placebo control to study the efficacy, while one study administered the drug for the same group of 40 patients in three periods comparing the change in viral load between the 3 time periods. All the studies included in the review showed a male preponderance.
The patients who received a higher dose of ibalizumab (15 mg/kg) showed an early significant rise in CD4+T-cell count at week 16 and week 48. The patients who received 10mg/kg of ibalizumab showed significant improvement in CD4 count at week 48 compared to placebo. The open-label trial that administered a maintenance dose of 6 mg/kg of ibalizumab every 2 weeks following a 10 mg/kg bolus showed a lesser increase in CD4 count when compared to the patients who received weekly 10 mg/kg ibalizumab.[4] The increase in CD4 count following ibalizumab administration was numerically lower in patients with pre-intervention CD4 count <50 cells/µL, though it was not statistically significant. The mean change in viral load was higher in the maintenance period when the patient received ibalizumab once in 2 weeks along with their optimized background regimen than during the functional monotherapy period.[6] The mean increase in CD4 cell count was found to be higher for the patients receiving weekly 10 mg/kg of ibalizumab compared to higher doses (25 mg/kg) or bolus doses of 10 mg/kg followed lower dose (6 mg/kg) at maintenance.[5]
The decrease in viral load in the double-blinded RCT was better in the arm receiving Ibalizumab 10 mg/kg than the arm receiving 15 mg/kg and placebo at all weeks of assessment (weeks 16, 24, and 48) but was statistically significant only on weeks 16 and 48.[4] In the multi-dose open-label trial, it was found that reduction of HIV-1 RNA viral copies was observed in patients harboring multi-drug-resistant HIV-1 even in the absence of other active anti-retroviral drugs. The studies showed sustained antiviral and immunological responses throughout the study duration, showing ≥0.5 log10 reduction in viral RNA copies compared to the baseline after the observation period.[4,6] After essential monotherapy with Ibalizumab, viruses were found to have reduced susceptibility to the drug in vitro.[5] Although the viral reduction after ibalizumab injection increases from the dose of 3 mg/kg, it was noted that beyond 10 mg/kg the viral load reduction was not increasing proportionately with 25 mg/kg.
It was observed in the studies that the adverse events reported were of mild-moderate severity. The serious/severe adverse events and deaths reported during the study were not found to be drug-related except for one IRIS (Immune reconstitution inflammatory syndrome).[6] Hence, the drug was well tolerated in all studies. It was also observed in the studies that many of the milder side effects observed were due to the concomitant use of ARTs. Although anti-ibalizumab antibodies were detected in a few patients, the significance remains unclear.[5]
This systematic review summarizes the role of ibalizumab in HIV-1 infected patients and compares the various dosages of ibalizumab and its efficacy based on CD4 count and HIV-1 viral load. It can be concluded that this medication has shown promising results in the treatment of HIV-1 infection, particularly in individuals who have developed resistance to multiple antiretroviral therapies. The reviewed studies consistently demonstrated the efficacy of ibalizumab in reducing viral load and increasing CD4 cell counts in treatment-experienced patients with multidrug-resistant HIV-1. In addition, ibalizumab was generally well-tolerated, with manageable side effects observed in the majority of patients. Thus, it opens the gate for further research in ibalizumab for naïve and drug-resistant HIV-1-infected patients.
CONCLUSION
Ibalizumab demonstrates promise as a therapeutic option for individuals with multidrug-resistant HIV-1. Its unique mechanism of action and positive impact on viral load reduction and CD4 cell counts make it a valuable addition to the armamentarium of HIV treatment options. Ongoing research and larger clinical trials will provide more comprehensive data and help establish the optimal role of ibalizumab in the management of HIV-1 infection.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. Vijayasankar Palaniappan, Dr. Kaliaperumal Karthikeyan, Dr. Reenaa Mohan are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Ibalizumab, a novel monoclonal antibody for the management of multidrug-Resistant HIV-1 infection. Antimicrob Agents Chemother. 2019;63:e00110-19.
- [CrossRef] [PubMed] [Google Scholar]
- Ibalizumab: A review in multidrug-resistant HIV-1 Infection. Drugs. 2020;80:189-96.
- [CrossRef] [PubMed] [Google Scholar]
- Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis. 2004;189:286-91.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy, pharmacokinetics, and safety over 48 weeks with ibalizumab-based therapy in treatment-experienced adults infected with HIV-1: A phase 2a study. J Acquir Immune Defic Syndr. 2021;86:482-9.
- [CrossRef] [PubMed] [Google Scholar]
- Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53:450-7.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379:645-54.
- [CrossRef] [PubMed] [Google Scholar]
- Deep sequencing of HIV: Clinical and research applications. Annu Rev Genomics Hum Genet. 2014;15:295-325.
- [CrossRef] [PubMed] [Google Scholar]
- Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009-2012. Clin Infect Dis. 2014;58:423-31.
- [CrossRef] [PubMed] [Google Scholar]
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort. PLoS Med. 2018;15:e1002537.
- [CrossRef] [PubMed] [Google Scholar]
- Strategies to overcome HIV drug resistance-current and future perspectives. Front Microbiol. 2023;14:1133407.
- [CrossRef] [PubMed] [Google Scholar]







