Translate this page into:
Clinicoradiological profile of idiopathic intracranial hypertension

*Corresponding author: Praveen Kumar Yadav, Department of Neurology, Aarogyam Neuroclinic, Durgapur, West Bengal, India. dr.praveen4u@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadav PK. Clinicoradiological profile of idiopathic intracranial hypertension. Glob J Health Sci Res 2023;1:121-5.
Abstract
Objectives:
Idiopathic intracranial hypertension (IIH) is a condition of raised intracranial pressure (ICP) in the absence of a space-occupying lesion. IIH patients usually present with typical symptoms and signs of increased ICP, such as headache, vomiting, neck pain, double vision, transient visual obscuration (TVO), and papilledema. Typical magnetic resonance imaging (MRI) findings include empty sella turcica, optic nerve tortuosity, globe flattening, and transverse sinus stenosis.
Material and Methods:
All patients from July 2021 to June 2022 presented to the super-specialty neuroclinic with symptoms suggestive of IIH were included in this study after consent. Clinical patterns and MRI brain findings along with other risk factors and comorbidities were studied.
Results:
Total 12 patients were studied out of which all were female. The most common age group was 31–40 years (41.6%). The most common clinical presentations were headache and TVOs followed by painless loss of vision, visual field changes and double vision. In this study, 7 out of 12 cases presented with headache (58.3%) out of which only 3 were migrainous. TVOs were presenting complaints of 7 patients (58.3%). Painless visual loss was there in 3 patients (25%), out of which only one case was bilateral (33%). In all cases (100%), MRI was suggestive of IIH. Transverse sinus stenosis in 3 cases (25%) and scleral flattening in 1 case (14.2%) were seen, respectively.
Conclusion:
Clinical suspicion followed by MRI brain is of utmost importance. Prompt diagnosis and treatment are essential in IIH patients to prevent permanent visual loss.
Keywords
Idiopathic
Intracranial hypertension
Cerebrospinal fluid
Magnetic resonance imaging
INTRODUCTION
Idiopathic intracranial hypertension (IIH) is characterized by an increase in intracranial pressure (ICP) without any identifiable etiology.[1,2] IIH is a rare disease, affecting 4.69/100,000 in the general population and the incidence of IIH is increasing with the increasing incidence of obesity.[3,4] The incidence of IIH is 20/100,000 in the obese population, with up to 64/100,000 in females.[5] IIH which was previously known as benign intracranial hypertension, is now classified under the umbrella term of pseudotumor cerebri syndrome with classification into primary or secondary depending on the presence or absence of any identifiable etiology.[6] According to the International Classification of Headache Disorders-3, IIH is described as a new-onset headache or significant worsening of a preexisting headache accompanied by clinical symptoms/signs, and/ or neuroimaging signs of raised increased ICP.[7] The majority of IIH patients are phenotypically obese females of reproductive age group where headache is the primary comorbidity.[1]
Aims
The aim of the study is to study the clinical presentation and radiological findings of IIH.
MATERIAL AND METHODS
This is a retrospective study, done at a super-specialty neuroclinic, in West Bengal, from July 2021 to June 2022. All the patients were examined by a single neurologist on an outpatient basis. Patients were enrolled in the study after proper consent.
Inclusion criteria
All patients with features of IIH (newly diagnosed and recurrent) between July 2021 and June 2022 were included in this study.
Exclusion criteria
Traumatic brain injury cases were excluded from the study.
After proper history taking, all patients underwent thorough neurological examination and then were followed up with brain imaging. Lumbar puncture and magnetic resonance imaging (MRI) brain were done in every case whereas magnetic resonance venography and MRI orbit was done in selected cases to rule out secondary causes of IIH such as venous sinus thrombosis. Age, sex, clinical presentation, and neuroimaging findings were studied retrospectively. All the patients enrolled in the study were treated afterwards.
All cases were female. Only two cases out of them were recurrent.
RESULTS
The most common clinical presentations were headache and transient visual obscurations (TVOs) followed by painless loss of vision, visual field (VF) changes and double vision. We found that 7 out of 12 cases were presented with headache (58.3%) out of which only 3were migrainous and the rest were non-migrainous. TVOs was presenting complaints of 7 patients (58.3%) with or without headache, 3 of them had no headache. Painless visual loss was there in 3 patients (25%), out of which only one case was bilateral (33%).
In all cases, MRI was suggestive of IIH with findings such as empty sella sign, dilated, and tortuous optic nerve. Transverse sinus stenosis in 3 cases and scleral flattening in 1 case were seen, respectively. On fundoscopy, papilledema was there in 4 cases where as one case had unilateral optic atrophy. The average cerebrospinal fluid (CSF) opening pressure was 31.25 cm of water. Two-fifth of cases were obese.
The most common age group of patients of IIH was 31–40 years [Table 1].
| Age group | Number of patients | Percentage |
|---|---|---|
| 21–30 | 3 | 25 |
| 31–40 | 5 | 41.66 |
| 41–50 | 4 | 33.33 |
IIH: Idiopathic intracranial hypertension
DISCUSSION
IIH, also known as primary pseudotumor cerebri, is characterized by increased ICP without any intracranial or spinal cord mass lesions and with normal-sized ventricles, normal CSF concentrations of protein and glucose, no cells in the CSF, and papilledema (Friedman et al., 2013[8,9] and modified Dandy criteria, 1985[10]).
IIH Diagnostic criteria; Friedman et al. 2013
Papilledema
Normal neurological examination (except sixth cranial nerve palsy)
Neuroimaging: normal brain parenchyma (no hydrocephalus, mass, structural lesion, or meningeal enhancement). Venous thrombosis was excluded in all
Normal CSF constituents
Elevated lumbar puncture pressure >25 cm CSF.
IIH was originally considered a benign syndrome of increased ICP with unremarkable imaging findings.[8,11] Over decades of research, it has been well understood that IIH is associated with characteristic imaging findings and has the potential of causing significant visual morbidity.[12,13] MRI is considered the gold standard investigation to exclude secondary causes of elevated ICP such as intracranial mass lesions, venous sinus thrombosis, and subarachnoid hemorrhage and to identify structural alterations associated with IIH such as empty sella turcica, flattening of the posterior optic globe, enlargement of the optic nerve sheath, and an increased tortuosity of the optic nerve.[14-18]
Over 90% of patients diagnosed with IIH experience headaches. Headache is the most common cause of morbidity and disability after the visual components of the disease are resolved.[3,19] The headache phenotype in IIH is predominantly migrainous, thus leading to a condition mimicking chronic migraine [Figures 1 and 2].[20] In addition to headache, clinical symptoms are mainly visual disturbances such as TVOs, VF loss and binocular diplopia – due to cranial nerve VI palsy and pulsatile tinnitus, back, and neck pain.[8,21] Most common VF defects are due to enlarged blind spots followed by loss of inferonasal portion of the VF.[6,22] Papilledema and raised ICP should be considered as the most severe stage of the spectrum whereas headache and pulsatile tinnitus could be considered as a benign form of IIH.[23] IIH is fulminant if the decline in visual function occurs abruptly within 4 weeks of diagnosis of IIH. IIH is said to be typical in the female of childbearing age with BMI >30 kg/m2 whereas in male and non-obese cases, more investigations are required to rule out secondary causes; hence described as atypical IIH.[24]
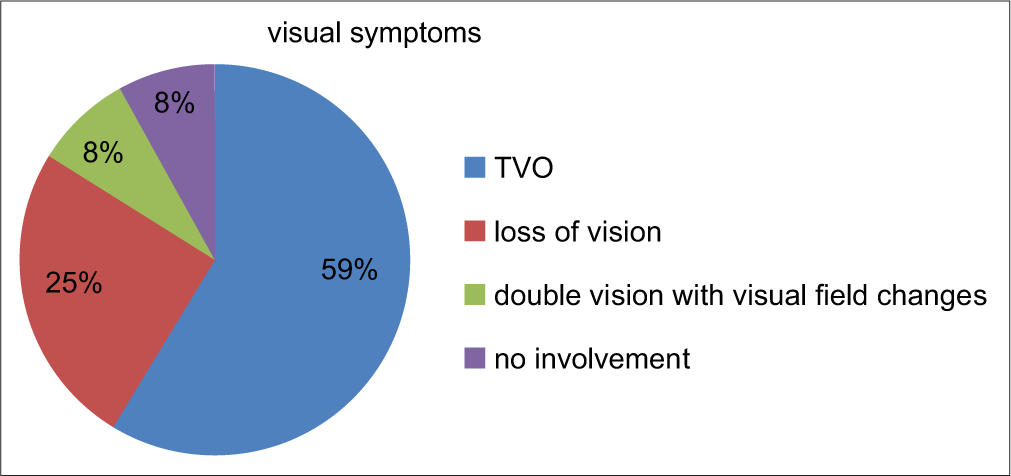
- Visual symptoms in the study population.

- Headache pattern and visual symptoms in patients with IIH
In our study, headache was presenting complaints in 58% of the cases whereas visual symptoms were present in 91% of cases out of which TVO was most common seen in 58% of cases. Loss of vision was there in 25% of cases and double vision was symptom in only 8% of the total case [Table 2]. According to the idiopathic intracranial hypertension treatment trial (IIHTT), headache was the most common symptom (84%), TVOs occurred in 68% of patients, diplopia in 22%, and pulse synchronous tinnitus in 52%. Only 32% reported visual loss.[25] In IIHTT, for CSF opening pressure of 200–250 mm water at least one of the following is required for diagnosis –pulse synchronous tinnitus, VIth nerve palsy, Frisen Grade II papilledema, echography for drusen negative and no other disc anomalies mimicking disc edema present, magnetic resonance venography with lateral sinus collapse/ stenosis preferably using auto-triggered elliptic centric-ordered technique, partially empty sella on coronal or sagittal views and optic nerve sheaths with filled out CSF spaces next to the globe on T2-weighted axial scans.[25]
| MRI findings | Number of cases | Percentage |
|---|---|---|
| Empty sella | 12 | 100 |
| Tortuous optic nerve | 12 | 100 |
| Transverse sinus stenosis | 2 | 16.66 |
| Scleral flattening | 1 | 8.33 |
IIH: Idiopathic intracranial hypertension, MRI: Magnetic resonance imaging
Less than half, that is, 42% of cases were obese [Figure 3]. All patients (100%) were female. In large studies, <10% of patients are male, for example, in IIHTT, only 2.4% were male.[25,26] In our study, the average CSF opening pressure was 31.25 cm of water [Table 3]. Only 1case (8.33%) was drug induced (levofloxacin).
| CSF opening pressure (in cm water) | Clinical features | MRI findings |
|---|---|---|
| 26–30 | TVO, double vision, non-migrainous headache | Empty sella, tortuous optic nerve |
| 31–35 | TVO, Unilateral loss of vision, migrainous headache | Transverse sinus stenosis, empty sella, tortuous optic nerve |
| 36–40 | Bilateral visual loss, migrainous headache | Scleral flattening, empty sella, tortuous optic nerve |
CSF: Cerebrospinal fluid, MRI: Magnetic resonance imaging, TVO: Transient visual obscuration

- Obese and Non-obese patients with IIH.
In our study, MRI findings such as empty sella, optic nerve tortuosity, and globe flattening were found in 100%, 100%, and 8% cases, respectively. The previous study by Agid et al. shows optic nerve tortuosity to be present in 40% of people with IIH and 8.9% of controls.[27,28] Agid et al. found empty sella to be present in 26.7% of people with IIH and 5.4% of controls whereas globe flattening was present in 43.3% of people with IIH and 0% of controls.[17,27] Absence of MRI findings does not rule out IIH.[27]
CONCLUSION
IIH is most prevalent in females of age group 31–40 years. Headache (58.3%) and/or TVOs (58.3%) are the most common presentation of IIH in our study. Clinical suspicion is of utmost importance for diagnosis of IIH to prevent complications. Every patient with headache and visual symptoms should undergo an MRI brain scan.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19:93.
- [CrossRef] [PubMed] [Google Scholar]
- Evolving evidence in adult idiopathic intracranial hypertension: Pathophysiology and management. J Neurol Neurosurg Psychiatr. 2016;87:982-92.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the link between obesity and headache-with focus on migraine and idiopathic intracranial hypertension. J Headache Pain. 2021;22:123.
- [CrossRef] [PubMed] [Google Scholar]
- The expanding burden of idiopathic intracranial hypertension. Eye (Lond). 2018;33:478-85.
- [CrossRef] [PubMed] [Google Scholar]
- Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol. 2019;76:1088-98.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic intracranial hypertension: The monster within. Ann Indian Acad Neurol. 2020;23:159-66.
- [Google Scholar]
- Headache classification committee of the international headache society (IHS) the international classification of headache disorders. In: Cephalalgia Vol 38. (3rd edition). 2018. p. :1-211.
- [CrossRef] [PubMed] [Google Scholar]
- Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-65.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated perioptic lipocalin-type prostaglandin D synthase concentration in patients with idiopathic intracranial hypertension. Brain Commun. 2022;4:fcac240.
- [CrossRef] [PubMed] [Google Scholar]
- Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2014;83:198-9.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic intracranial hypertensionthe eyes and beyond. Ann Indian Acad Neurol. 2022;25:179-80.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity and specificity of neuroimaging signs in patients with idiopathic intracranial hypertension. Neuroradiol J. 2021;34:421-7.
- [CrossRef] [PubMed] [Google Scholar]
- Factors affecting visual field outcomes in the idiopathic intracranial hypertension treatment trial. J Neuroophthalmol. 2016;36:6-12.
- [CrossRef] [PubMed] [Google Scholar]
- Volumetric assessment of optic nerve sheath and hypophysis in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2014;35:513-8.
- [CrossRef] [PubMed] [Google Scholar]
- Narrowing of Meckel's cave and cavernous sinus and enlargement of the optic nerve sheath in pseudotumor cerebri. J Comput Assist Tomogr. 2011;35:308-12.
- [CrossRef] [PubMed] [Google Scholar]
- Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia. 2013;33:1075-84.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic intracranial hypertension: The validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudotumor cerebri: Brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32:1986-93.
- [CrossRef] [PubMed] [Google Scholar]
- Headache determines the quality of life in idiopathic intracranial hypertension. J Headache Pain. 2015;16:4.
- [CrossRef] [PubMed] [Google Scholar]
- Headache attributed to idiopathic intracranial hypertension and persistent post-idiopathic intracranial hypertension headache: A narrative review. Headache. 2021;61:808-16.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated perioptic lipocalin-type prostaglandin D synthase concentration in patients with idiopathic intracranial hypertension. Brain Commun. 2022;4:fcac240.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991;114:155-80.
- [Google Scholar]
- Idiopathic intracranial hypertension: Glymphedema of the brain. J Neuroophthalmol. 2021;41:93-7.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic intracranial hypertension: Consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088-100.
- [CrossRef] [PubMed] [Google Scholar]
- The idiopathic intracranial hypertension treatment trial: Clinical profile at baseline. JAMA Neurol. 2014;71:693-701.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and risk factors for idiopathic intracranial hypertension. Int Ophthalmol Clin. 2014;54:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- MRI findings as markers of idiopathic intracranial hypertension. Curr Opin Neurol. 2021;34:75-83.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of papilledema and visual pathways: Effects of increased intracranial pressure and pathophysiologic mechanisms. AJNR Am J Neuroradiol. 2012;34:919-24.
- [CrossRef] [PubMed] [Google Scholar]







