Translate this page into:
Dipstick urinalysis and antibiogram of bacteria isolates from asymptomatic undergraduate male students

*Corresponding author: Ijeoma Ngozi Ebenebe, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Agulu, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria. in.ebenebe@unizik.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Igbokwe NH, Uzor IB, Osuala OJ, Ebenebe IN, Oli AN. Dipstick urinalysis and antibiogram of bacteria isolates from asymptomatic undergraduate male students. Glob J Health Sci Res. doi: 10.25259/GJHSR_23_2025
Abstract
Objectives:
The study aims to determine the prevalence of asymptomatic bacteriuria in male students and evaluate the effectiveness of dipstick urinalysis in detecting bacteriuria.
Material and Methods:
A total of 312 undergraduate male students in the university across different departments within the age of 18–37 years old, who fulfilled the inclusive criteria for the study were sampled. Approximately 20–30 mL of midstream urine was collected from each participant in sterile, labeled containers, and transported to the laboratory within 2–4 h of collection. Dipstick urinalysis was carried out on each of the urine samples using Accu-Answer® dipsticks according to the manufacturer’s instructions. Parameters measured included pH, protein, glucose, nitrites, urobilinogen, blood, ketone, bilirubin, and ascorbic acid. Urine samples were aseptically cultured on appropriate media to isolate bacterial strains and characterized them using standard microbiological and biochemical methods. The antibiotic susceptibility profile of the isolates was determined using the Kirby– Bauer disk diffusion method.
Results:
Results obtained from the dipstick urinalysis were recorded for each participant, and any abnormal findings flagged for further bacteriological analysis. A total of 164 bacterial isolates were obtain from the samples with 90 Staphylococcus aureus having a prevalence of 54.87%, followed by 39 Salmonella typhi having a prevalence of 23.78%, with 28 Escherichia coli and 7 Shigella dysenteriae having a prevalence of 17.07% and 4.26%, respectively. Antibiotic susceptibility testing revealed that all of the isolates were resistant to most of the antibiotics that they were tested with. S. aureus and Salmonella typhi strains which showed between 45.1–89.0% and 43.5–100% resistance to all the antibiotic disc tested, respectively. However, both E. coli and S. dysenteriae had the highest level of resistance with the majority of the antibiotics disc having 100%, aspect for few.
Conclusion:
The antibiotic resistance of these isolates calls for concern.
Keywords
Antibiogram and antibiotic resistance
Asymptomatic bacteriuria
Dipstick
Urinalysis
INTRODUCTION
Asymptomatic bacteriuria (ASB) is the presence of a significant quantity of bacteria in the urine, typically above 105 colony-forming units per milliliter, without the clinical symptoms associated with urinary tract infections (UTIs).[1] Although ASB often remains benign, it can pose a risk for progression to symptomatic UTIs or even more severe complications, such as pyelonephritis or sepsis, if left untreated in vulnerable populations.[2] Young male adults are generally considered to be at a lower risk for UTIs compared to females, due to anatomical and physiological factors. However, recent studies have suggested that the prevalence of ASB in asymptomatic males, particularly in institutional settings such as universities, may be higher than previously thought.[3] This population is often subjected to a range of stressors and lifestyle habits, including inadequate hydration, irregular personal hygiene, and prolonged sitting periods, all of which may predispose them to urinary stasis and subsequent bacteriuria.[4] Early detection and appropriate management of ASB in young, healthy individuals are crucial for preventing the potential spread of drug-resistant bacterial strains within the community. Dipstick urinalysis is a rapid, non-invasive, critical initial step, and cost-effective tool used to detect indicators of infection and other urological conditions, including nitrites, leukocyte esterase, glucose, ketones, and protein in the urine.[1,5] This test can serve as an initial step in the identification of potential ASB cases, allowing for timely interventions to be made. However, despite its convenience and affordability, dipstick urinalysis has limitations, particularly regarding sensitivity and specificity, making it essential to confirm results with culture-based techniques. An antibiogram, which tests the susceptibility of bacterial isolates to a range of antibiotics, is another essential tool in the management of ASB. The growing threat of antibiotic resistance underscores the importance of conducting regular antibiogram assessments to guide effective treatment protocols.[6] In cases of ASB, understanding the resistance profile of uropathogens is critical, as the overuse or misuse of antibiotics can lead to the development of multidrug-resistant organisms (MDROs). Recent reports suggest that antibiotic resistance, particularly to commonly used agents such as ciprofloxacin (CIP), trimethoprimsulfamethoxazole (SXT), and amoxicillin-clavulanate, is rising among uropathogens isolated from ASB cases in young adults.[7]
MATERIAL AND METHODS
Study area
The study was conducted in Madonna University Elele campus. Elele is one of the big towns in Ikwerre local government area of rivers state, south-south, Nigeria.
Study population
The study population include male students aged 18–37 years in Madonna university, Elele, Rivers State.
Study size
The calculated sample size of 310 was estimated using Yarmane’s[8] formula:
Where n = desired sample size
e = maximum acceptable margin of error, set at 0.05
N = population
1 = a theoretical constant.
Then adding 10% attrition
Using the formula, the sample size (n) was 312.
Study design
This descriptive cross-sectional study is aimed at evaluating the prevalence of ASB and the effectiveness of dipstick urinalysis among asymptomatic male university students. In addition, bacteriological analysis will be conducted to identify common urinary pathogens and their antimicrobial resistance (AMR) patterns.
Sampling technique
Participants will be selected using a stratified random sampling method to ensure a representative sample across different faculties within the university. This method will ensure that the sample is diverse and reflects the demographic characteristics of the broader student population.
Inclusion and exclusive criteria
Male university students aged 18–37 years and asymptomatic individuals (no symptoms of UTIs such as dysuria, frequency, urgency, or fever) will be included. Participants with a history of UTIs in the past 3 months, individuals currently on antibiotic therapy, and those with known chronic conditions, such as diabetes, renal disease, or immunosuppression will be excluded.
Ethical considerations
This study was conducted in accordance with ethical principles for medical research involving human subjects. Before sample collection, participants will be required to provide informed consent after being fully briefed on the study’s purpose and procedures. All information collected from the participants was kept confidential, with results used exclusively for research purposes. The study protocol was reviewed and approved by Madonna University’s Ethical Review Board (Ethics Committee) before the study was commenced. Approval number: MAU/DRC/HD/E/PHARM/2024/056.
Sample collection
Urine samples were collected in the morning from asymptomatic male students following informed consent, with instructions for midstream collection to minimize contamination.
Approximately 20–30 mL of midstream urine was collected from each participant in sterile, labeled containers, and transported to the laboratory within 2–4 h of collection.
Urine analysis using dipstick (dipstick urinalysis)
According to the manufacturer’s instructions, each urine sample was analyzed using Accu-Answer® dipsticks. Parameters measured included pH, protein, glucose, nitrites, urobilinogen, blood, ketone, bilirubin, and ascorbic acid.
Results were recorded for each participant, and any abnormal findings were flagged for further bacteriological analysis.
Bacteriological analysis
Culturing and isolation
Urine samples were aseptically inoculated on nutrient agar, eosin methylene blue (EMB) agar, Mannitol Salt agar, Cetrimide agar, and Shigella-Salmonella agar plates using a spread plate method. Plates were incubated at 37°C for 18–24 h.
Identification of bacterial isolates
After incubation, bacterial colonies were examined for morphological characteristics. Gram staining was performed to classify isolates as Gram-positive or Gram-negative. Biochemical tests, including catalase, coagulase, oxidase, indole, and motility tests, were used for further identification.
Antibiotic sensitivity testing
Antibiotic susceptibility testing (AST) is usually carried out to determine the susceptibility pattern of the test organisms. Testing for antibiotic sensitivity of the clinical isolates (test organisms) was done using the Kirby–Bauer method.[9] Multiple antibiotic disc containing different antibiotics: Ofloxacin (OFX, 5 µg); Ciprofloxacin (CIP 5 µg); Amoxycillin-Clavulanic acid (AMC, 20/10 µg); Gentamicin, (GEN, 10 µg); Ceftazidime (CAZ, 30 µg); Cefotaxime (CTX, 30 µg); Trimethoprim-Sulfamethoxazole (SXT 1.25/23.75 µg); Ampicillin (AMP, 10 µg); Tetracycline (TE, 30 µg); and Ceftriaxone (CRO, 30 µg) ; Azithromycin (AZN, 15 µg); Nalidixic acid (NA, 30 µg); Ampicillin+Cloxacillin (ACX, 10 µg); Nitrofurantoin (NF, 300 µg) with specific concentrations was placed onto a sterile agar plate (Muёller-Hinton Agar) on which the test isolates, standardized to 0.5 McFarland are growing.
The plates were inverted and left on the work table for 30 min to allow for pre-diffusion of antibiotics into the agar. The plates were incubated at 37°C for 18–24 h. The susceptibility of each isolate to each antibiotic was shown by a clear zone of growth inhibition and this was measured using a meter rule in millimeters and the diameter of the zones of inhibition was then interpreted using a standard chart Clinical and Laboratory Standards Institute (CLSI) guidelines to determine susceptibility or resistance profiles.
If the test isolates are sensitive to the antibiotic, a clear ring, or zone of inhibition is seen around that disc indicating sensitivity whereas if no observable clear ring is seen around that disc, it indicates resistance.
Data analysis
Data obtained from dipstick urinalysis and bacteriological findings were statistically analyzed using the Statistical Package for the Social Sciences software. Descriptive statistics, such as frequencies and percentages, were used to summarize the prevalence of bacteriuria and dipstick results.
RESULTS
Table 1 summarizes the demographics of the participants across the various level of study with different age groups, lacking statistically significant results, with P-values exceeding 0.05 across all categories. Thus, this indicates no strong association between these demographics and the presence of isolates, while Table 2 summarizes the relationship between age groups and year of study with the presence or absence of bacteriuria. No significant correlation was observed, with P-values exceeding 0.05 across all categories.
| Values recorded | Chi-square | P-value | |
|---|---|---|---|
| Age | |||
| <18–23 years | 224 | 3.505 | 0.173 |
| 23–27 years | 80 | 0.125 | 0.723 |
| 28–32 years | 8 | 1.510 | 0.220 |
| 33–37 years | 0 | 0 | 1.000 |
| >37 years | 0 | 0 | 1.000 |
| Total | 312 | ||
| Year of study | |||
| 100 level | 32 | 0.712 | 0.399 |
| 200 level | 68 | 1.327 | 0.249 |
| 300 level | 83 | 0.249 | 0.619 |
| 400 level | 68 | 0.153 | 0.696 |
| 500 level | 40 | 0.056 | 0.813 |
| 600 level | 21 | 0.076 | 0.783 |
| Total | 312 | ||
| Bacteriuria present | Bacteriuria absent | Total | Chi-square | P-value | |
|---|---|---|---|---|---|
| Age group (years) | |||||
| <18–23 | 201 | 23 | 224 | 0.324 | 0.568 |
| 23–27 | 70 | 10 | 80 | 0.025 | 0.423 |
| 28–32 | 6 | 2 | 8 | 0.422 | 0.421 |
| 33–37 | 0 | 0 | 0 | 0 | 1.000 |
| >37 | 0 | 0 | 0 | 0 | 1.000 |
| Total | 277 | 35 | 312 | ||
| Year of study (level) | |||||
| 100 | 30 | 2 | 32 | 0.232 | 0.347 |
| 200 | 57 | 11 | 68 | 0.456 | 0.267 |
| 300 | 76 | 7 | 83 | 0.723 | 0.221 |
| 400 | 60 | 8 | 68 | 0.632 | 0.216 |
| 500 | 35 | 5 | 40 | 0.311 | 0.121 |
| 600 | 19 | 2 | 21 | 0.032 | 0.34 |
| Total | 277 | 35 | 312 | ||
Percentage prevalence
Table 3 presents the distribution and prevalence of the four main bacterial isolates identified in the study: Staphylococcus aureus, Escherichia coli, Salmonella typhi, and Shigella dysenteriae. The table shows the total number of isolates for each organism, as well as the percentage of each isolate relative to the total number of isolates . S. aureus is the most prevalent organism, comprising 55.15% of the total isolates, followed by Salmonella typhi at 23.78%, E. coli at 17.07%, and S. dysenteriae at 4.26%. The “Prevalence rate %” column provides the proportion of participants with each respective organism, indicating how common each pathogen is within the study population.
| Code | Isolates | Number of isolates | Prevalence rate % |
|---|---|---|---|
| A | Staphylococcus aureus | 90 | 54.87 |
| B | Escherichia coli | 28 | 17.07 |
| C | Salmonella typhi | 39 | 23.78 |
| D | Shigella dysenteriae | 7 | 4.26 |
| Total | 164 | 100 |
Dipstick urinalysis
The findings of dipstick urinalysis for different departments are shown in Table 4, which indicates whether or not, certain urine parameters were present in study participants. Including the number of participants in each department and their associated result parameters such nitrates, urobilinogen, protein, pH, blood, ketone, bilirubin, glucose, and ascorbic acid.
| Parameters | Departments with positive results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLS (31) | PHARM (50) | BCH (16) | OPT (31) | MD (40) | CS (37) | PHY (17) | ANT (9) | NSC (37) | MCB (7) | PH (28) | IC (9) | |
| Nitrates | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urobilinogen | 0 | 5 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Protein | 5 | 1 | 1 | 2 | 4 | 3 | 1 | 1 | 4 | 2 | 4 | 0 |
| pH | 5 | 3 | 2 | 6 | 2 | 3 | 2 | 0 | 2 | 0 | 2 | 0 |
| Blood | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ketone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bilirubin | 10 | 22 | 2 | 3 | 2 | 1 | 3 | 1 | 4 | 1 | 3 | 0 |
| Glucose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ascorbic acid | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
MLS: Medical laboratory science, PHARM: Pharmacy, BCH: Biochemistry, OPT: Optometry, MD: Medicine and surgery, CS: Computer science, PHY: Physiology, ANT: Anatomy, NSC: Nursing science, MCB: Microbiology, PH: Public health, IC: Industrial chemistry
Morphological and biochemical characteristics of isolates
Table 5 describes the morphological features of each bacterial isolate based on Gram staining and biochemical reactions. The distinctions are important for determining the bacterial type and to give guidance for further testing procedures, as Gram-negative bacteria are often associated with increased health risks.
| Parameter / Unknown Isolates | A | B | C | D |
|---|---|---|---|---|
| Cultural characteristics | It gave a yellow coloration when streaked on a Mannitol Salt Agar | It forms pink coloration on MacConkey Agar | It forms a colorless colony with or without black centers on Salmonella-Shigella agar | Forms small, colorless colonies as it does not ferment lactose or produce H2S on Salmonella-Shigella agar |
| Gram staining | Gram-positive Cocci | Gram-negative Rod | Gram-negative Rod | Gram-negative Rod |
| Biochemical test | ||||
| Citrate | + | - | + | - |
| Oxidase | - | - | - | - |
| Indole | - | - | - | + |
| Motility | - | + | + | - |
| Coagulase | + | - | - | - |
| Catalase | + | + | + | + |
| Suspected organism | Staphylococcus aureus | E. coli | Salmonella typhi | Shigella dysenteriae |
Respondents and organisms isolated
Table 6 shows the clinical and demographic details of the study participants from each department, including the proportion of people who had microbial growth and those who did not. The information shows how participants from various departments were distributed in terms of isolated organisms, such as Shigella, Salmonella, E. coli, and Staphylococcus. Alongside the matching types and number of organisms isolated. The total number of participants in each department and the number of participants with positive microbial growth are also revealed. A summary of the pathogens’ overall prevalence in each department is also included in the table, which sheds light on the variety of microbial isolates in respect to the participant demographics. This data is essential for comprehending microbial colonization patterns and possible correlations with different department.
| Departments | Study participants | Number of participants with growth | Number of participants with growth | Number of organisms isolated |
|---|---|---|---|---|
| MLS | 31 | 17 | 14 | Staphylococcus (12), E. coli (2), Salmonella (4), Shigella (0) |
| Pharmacy | 50 | 23 | 27 | Staphylococcus (15), E. coli (5), Salmonella (4), Shigella (2) |
| BCH | 16 | 9 | 7 | Staphylococcus (7), E. coli (1), Salmonella (2), Shigella (0) |
| OPT | 31 | 14 | 17 | Staphylococcus (9), E. coli (3), Salmonella (3), Shigella (1) |
| Medicine | 40 | 11 | 29 | Staphylococcus (7), E. coli (1), Salmonella (3), Shigella (1) |
| Computer science | 37 | 16 | 21 | Staphylococcus (9), E. coli (3), Salmonella (5), Shigella (1) |
| Physiology | 17 | 8 | 9 | Staphylococcus (5), E. coli (1), Salmonella (4), Shigella (0) |
| Anatomy | 9 | 5 | 4 | Staphylococcus (4), E. coli (2), salmonella (2), shigella (0) |
| Nursing | 37 | 16 | 21 | Staphylococcus (8), E. coli (5), Salmonella (4), Shigella (1) |
| Microbiology | 7 | 5 | 2 | Staphylococcus (4), E. coli (2), salmonella (1), shigella (0) |
| Public health | 28 | 12 | 16 | Staphylococcus (6), E. coli (2), Salmonella (6), Shigella (1) |
| Industrial chemistry | 9 | 5 | 4 | Staphylococcus (4), E. coli (1), Salmonella (1), Shigella (0) |
| Total | 312 | 141 | 171 | Staphylococcus (90) E. coli (28), Salmonella (39), Shigella (7) |
MLS: Medical laboratory science, BCH: Biochemistry, OPT: Optometry
Antimicrobial Susceptibility results of the isolates
Table 7 presents the antimicrobial susceptibility profile of S. aureus isolates according to the CLSI breakpoints for different antibiotics. The table categorizes the isolates as susceptible (S), intermediate (I), or resistant (R) to a range of antibiotics, providing the percentage of isolates in each category. The data reveals notable resistance patterns across various antibiotics.
| Antibiotics | S | % | I | % | R | % |
|---|---|---|---|---|---|---|
| Amoxicillin+clavulanate | 5 | 5.4 | 5 | 5.4 | 81 | 89.0 |
| Cefotaxime | 30 | 32.9 | 9 | 9.8 | 52 | 57.1 |
| Ceftriaxone sulbactam | 20 | 21.9 | 6 | 6.5 | 65 | 71.4 |
| Imipenem/cilastatin | 28 | 30.7 | 6 | 6.5 | 57 | 62.6 |
| Cefuroxime | 13 | 14.2 | 7 | 7.6 | 71 | 78.0 |
| Ofloxacin | 39 | 42.8 | 11 | 12.1 | 41 | 45.1 |
| Cefixime | 34 | 37.3 | 8 | 8.7 | 49 | 53.8 |
| Levofloxacin | 41 | 45.1 | 9 | 9.8 | 41 | 45.1 |
| Ciprofloxacin | 30 | 32.9 | 11 | 12.1 | 50 | 54.9 |
| Erythromycin | 27 | 29.6 | 12 | 13.1 | 52 | 57.1 |
| Gentamycin | 15 | 16.4 | 6 | 6.5 | 70 | 76.9 |
| Azithromycin | 14 | 15.3 | 6 | 6.5 | 71 | 78.0 |
S: Susceptibility, I: Intermediate, R: Resistant
Table 8 reveals the antimicrobial susceptibility profiles for the three predominant Gram-negative bacterial isolates : E. coli, Salmonella typhi, and S. dysenteriae. The table presents the percentage of isolates categorized as susceptible (S), intermediate (I), or resistant (R) to a range of antibiotics.
| Antibiotics | (n=28) | typhi (n=39) | (n=7) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | % | I | % | R | % | S | % | I | % | R | % | S | % | I | % | R | % | |
| ACX | 0 | 0 | 0 | 0 | 28 | 100 | 8 | 20.5 | 2 | 5.1 | 29 | 74.3 | 0 | 0 | 0 | 0 | 7 | 100 |
| CTX | 0 | 0 | 0 | 0 | 28 | 100 | 0 | 0 | 0 | 0 | 39 | 100 | 0 | 0 | 0 | 0 | 7 | 100 |
| IMP | 0 | 0 | 0 | 0 | 28 | 100 | 3 | 7.6 | 2 | 5.1 | 34 | 87.1 | 0 | 0 | 3 | 42.8 | 4 | 57.1 |
| OFX | 8 | 28.5 | 3 | 10.7 | 17 | 60.7 | 4 | 10.2 | 3 | 7.6 | 32 | 82.1 | 5 | 71.4 | 0 | 0 | 2 | 28.5 |
| ZEM | 0 | 0 | 1 | 3.5 | 27 | 96.4 | 7 | 17.9 | 5 | 12.8 | 27 | 69.2 | 2 | 28.5 | 3 | 42.8 | 2 | 28.5 |
| NA | 0 | 0 | 0 | 0 | 28 | 100 | 8 | 20.5 | 6 | 15.3 | 25 | 64.1 | 0 | 0 | 0 | 0 | 7 | 100 |
| CRO | 0 | 0 | 0 | 0 | 28 | 100 | 2 | 5.1 | 3 | 7.6 | 34 | 87.1 | 0 | 0 | 0 | 0 | 7 | 100 |
| CXM | 0 | 0 | 0 | 0 | 28 | 100 | 2 | 5.1 | 2 | 5.1 | 35 | 89.7 | 0 | 0 | 0 | 0 | 7 | 100 |
| LBC | 9 | 32.1 | 4 | 14.3 | 15 | 53.5 | 17 | 43.5 | 5 | 12.8 | 17 | 43.5 | 3 | 42.8 | 2 | 28.5 | 2 | 28.5 |
| GN | 1 | 3.5 | 2 | 7.1 | 25 | 89.2 | 9 | 23.1 | 5 | 12.8 | 25 | 64.1 | 2 | 28.5 | 1 | 14.2 | 4 | 57.1 |
| AUG | 0 | 0 | 0 | 0 | 28 | 100 | 2 | 5.1 | 0 | 0 | 37 | 94.8 | 0 | 0 | 0 | 0 | 7 | 100 |
| NF | 3 | 10.7 | 3 | 10.7 | 22 | 78.5 | 15 | 38.4 | 1 | 2.5 | 23 | 58.9 | 0 | 0 | 3 | 42.8 | 4 | 57.1 |
S: Susceptibility, I: Intermediate, R: Resistant, ACX: Ampiclox, CTX: Cefotaxime, IMP: Imipenem, OFX: Ofloxacin, ZEM: Cefexime, NA: Nalidixic acid, CRO: Ceftriaxone sulbactam, CXM: Cefuroxime, LBC: Levofloxacin, GN: Gentamycin, AUG: Amoxicillin clavulanate, NF: Nitrofurantoin
Multiple antibiotic resistance (MAR) index S. aureus
Figure 1 below provides an overview of the MAR index patterns observed among the isolate S. aureus calculated by dividing the number of antibiotics to which the strain is resistant by the total number of antibiotics tested with indices ranging from 0.08 to 0.83.
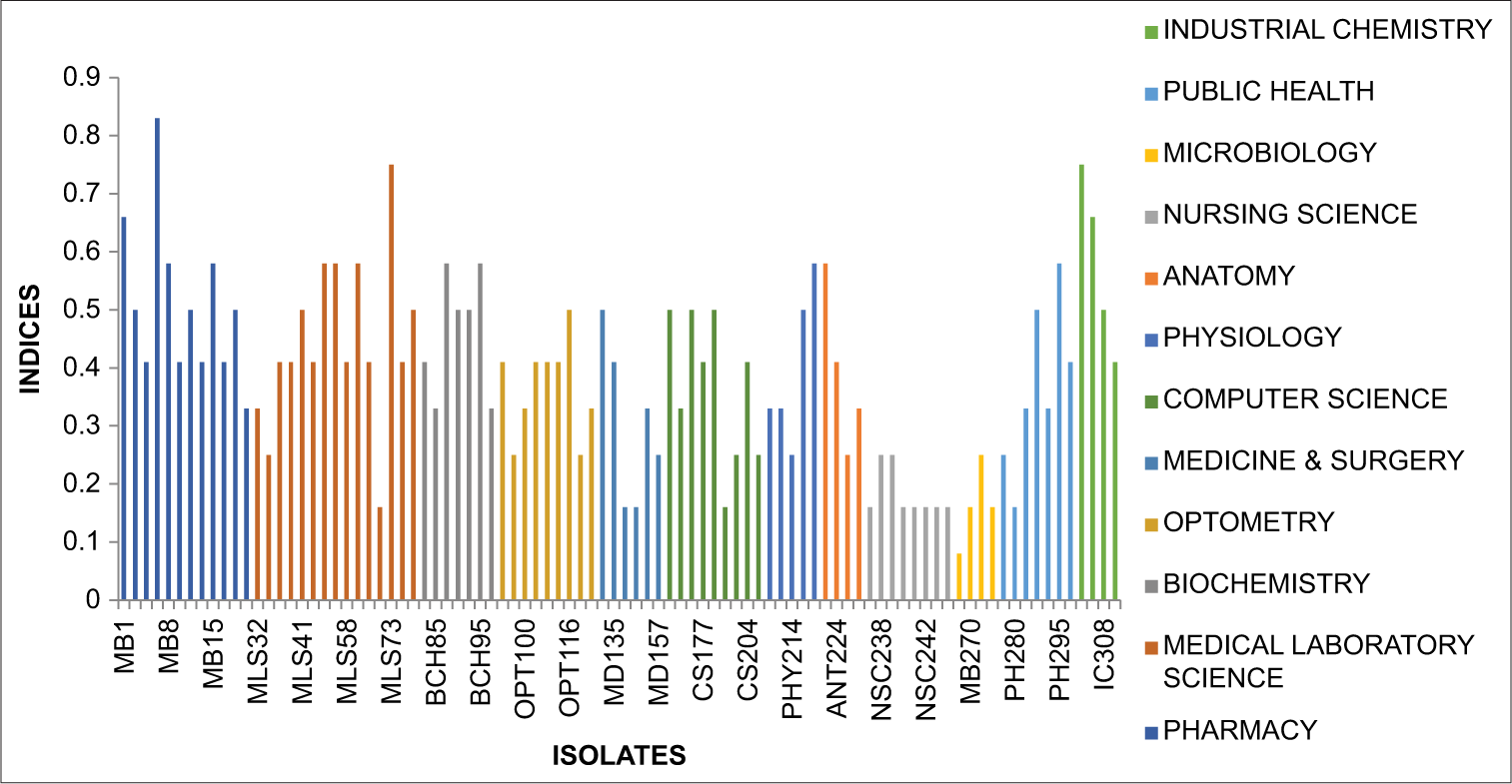
- Graph showing multiple antibiotic resistance index Staphylococcus aureus.
MAR index E. coli
Figure 2 below provides an overview of the MAR index patterns observed among the isolate E. coli with indices ranging from 0.00 to 0.33.
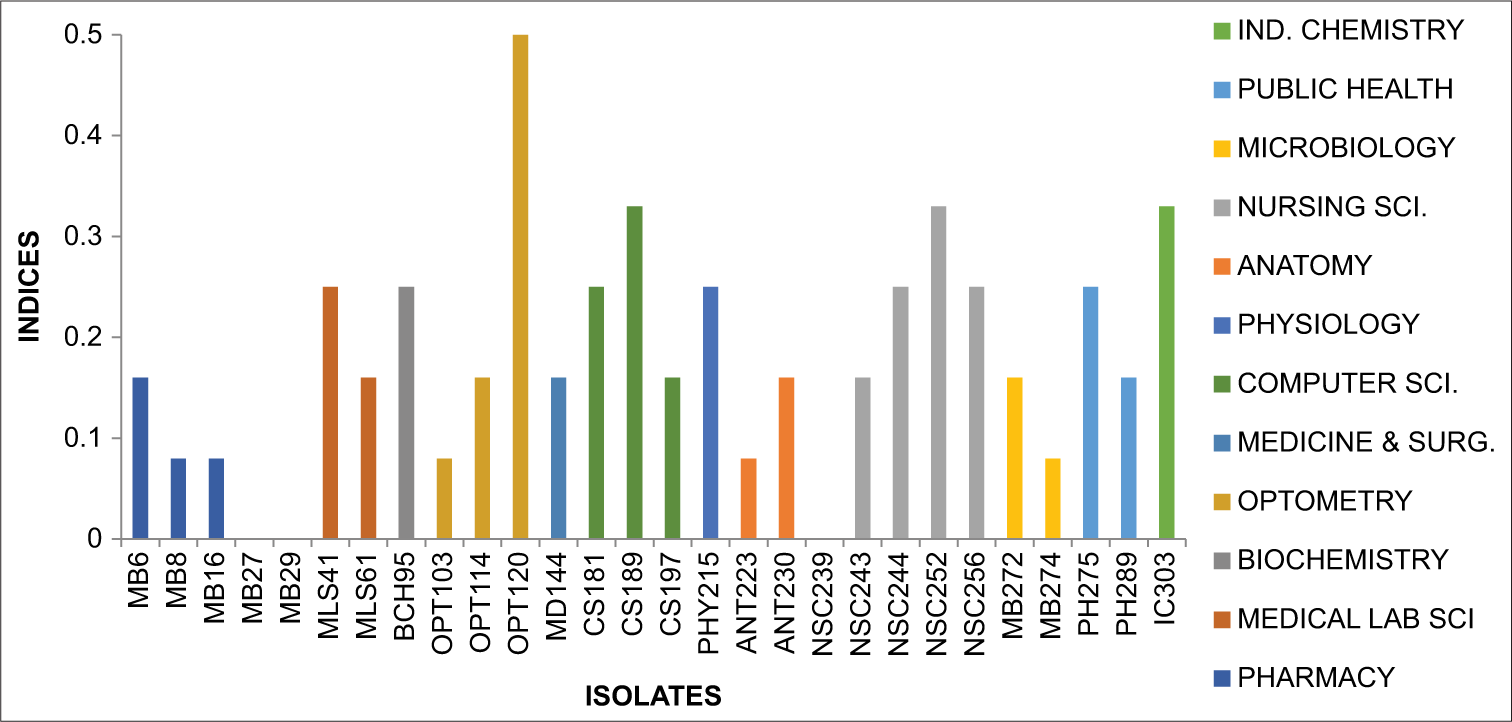
- Graph showing multiple antibiotic resistance index Escherichia coli.
MAR index Salmonella typhi
Figure 3 below provides an overview of the MAR index patterns observed among the isolate (Salmonella typhi ) with indices ranging from 0.08 to 0.58.
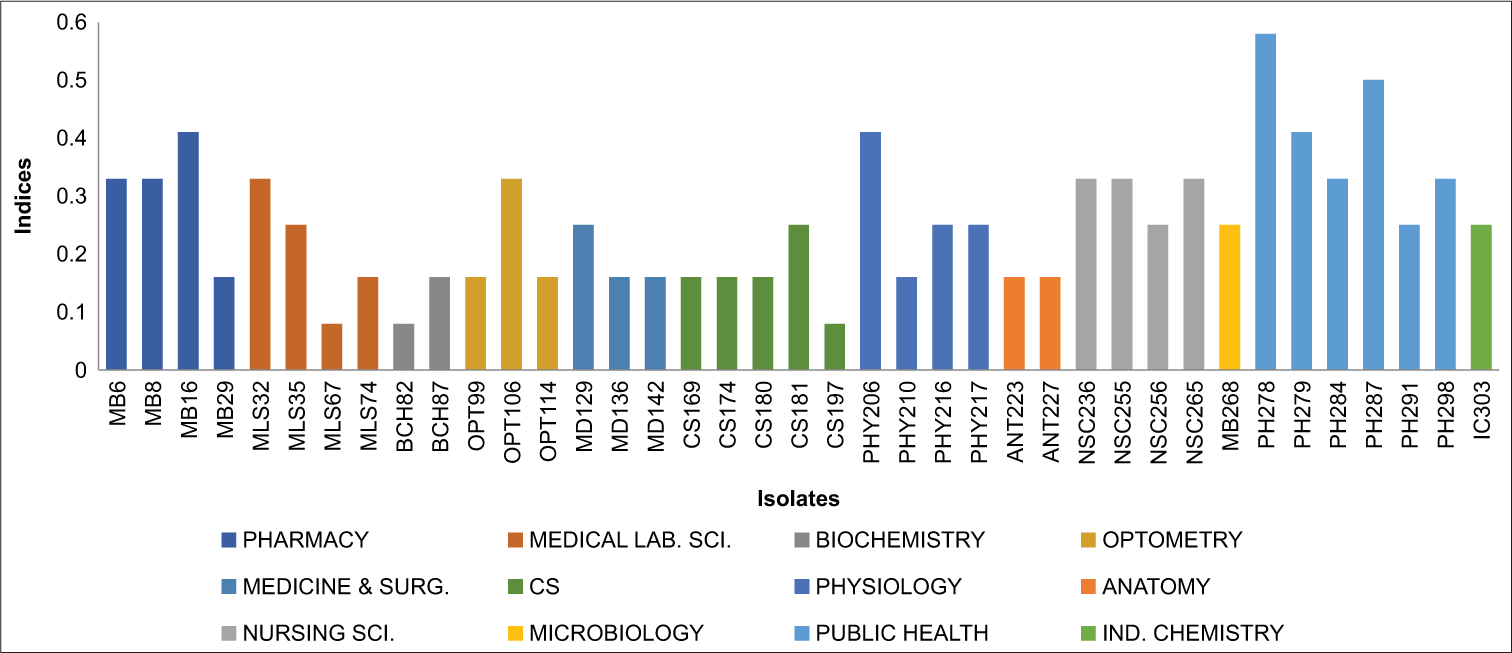
- Graph showing multiple antibiotic resistance index Salmonella typhi.
MAR index S. dysenteriae
Figure 4 below provides an overview of the MAR index patterns observed among the isolate (S. dysenteriae) with indices ranging from 0.25 to 0.58.
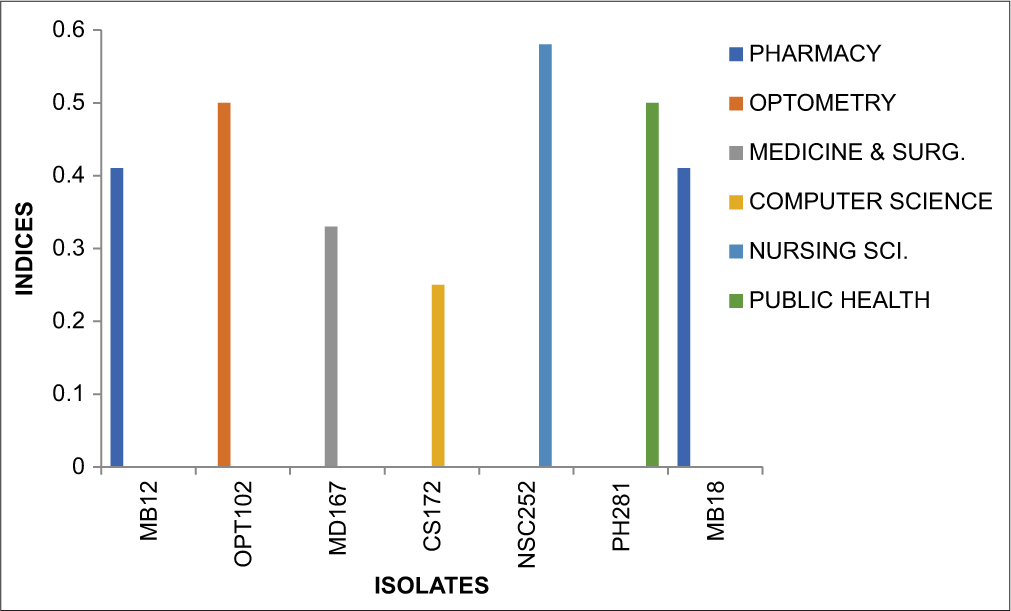
- Graph showing multiple antibiotic resistance Index Shigella dysenteriae.
DISCUSSION
Bacteriuria has been discovered to pave the way for colonizing specific species of bacteria that produce UTIs. ASB is known to promote the significant spread of multi-drug-resistant pathogens. However, different microbes are known to infect and cause bacteriuria.[10-12] The prevalence of ASB in the study was 89.4% with S. aureus being the most prevalent organism, comprising 54.87% of the total isolates, followed by Salmonella typhi at 23.78%, E. coli at 17.07%, and S. dysenteriae at 4.26%. The most prevalent organism reported in this study is known to be a commensal organism found commonly in skin microflora and mucous membranes. It is known to cause opportunistic infections mostly among immunocompromised individuals. It has been reported to cause bloodstream, skin, and soft-tissue infections including UTI.[13-15] A similar, high prevalence rate of S. aureus has been reported in previous studies.[16-19] However, results from previous studies that are at variance with the present study have been reported.[20,21] The high prevalence of S. aureus can be ascribed to its high tolerance to pH and temperature variations, thus having a greater ability to colonize different areas of the body.[22] Antibiotic susceptibility profile was also recorded for S. aureus with susceptibility to levofloxacin being 45.1% and high resistance was recorded in amoxicillin clavulanate 89%, gentamycin 78%, and azithromycin 78%. E. coli also showed susceptibility to levofloxacin (32.1%) and OFX (28.1%) but had the least susceptibility to amoxicillin clavulanate (0%) and ampiclox (0%). Salmonella typhi showed susceptibility to levofloxacin (43.5%) and nitrofurantoin (38.4%), and showed significant resistance to CTX (100%), amoxicillin-clavulanate (94.8%), and cefuroxime (89.7%) among others. While for S. dysenteriae relative susceptibility was recorded in OFX (71.4%) and levofloxacin (42.8%), there was significant high resistance recorded in ampiclox, CTX, nalidixic acid, CRO sulbactam, and cefuroxime with each being at a 100%.
The results of this study on the antibiogram profiles and dipstick urinalysis of male undergraduate students at Madonna University, Elele, who were asymptomatic, offer important new information about the frequency of bacterial infections and urine abnormalities in this population. A thorough understanding of urinary health and microbial resistance trends in an academic setting can be obtained from the results of the dipstick urinalysis, bacteriological analysis, and AST that follow.
The presence of distinct urine abnormalities across departments was shown by the dipstick urinalysis, which illustrates the range of physiological states and potential risk factors among students. Notably, proteinuria was found across a number of fields, with medicine and surgery and medical laboratory science (MLS) having the highest prevalence. Although asymptomatic proteinuria may also be benign, protein in the urine may be a sign of renal pathology, such as glomerulonephritis or UTIs.[23] Although more research is needed to determine the clinical importance in asymptomatic persons, the presence of bilirubin in the urine was more common in pharmacies and may indicate hemolytic disorders or liver failure. Interestingly, ketones and glucose were absent in every area, which is in line with the research population’s lack of obvious metabolic conditions such as diabetes or ketosis. In addition, no nitrates were found in any of the samples, suggesting that asymptomatic students did not frequently get UTIs from nitrate-reducing bacteria (such as E. coli). Nevertheless, several participants had urobilinogen, although in trace amounts. The modest levels of urobilinogen detected here are probably not clinically significant and may be related to the physiological variability of asymptomatic persons, but elevated levels may indicate hemolysis or liver illness. In terms of the relationship between individual departments and bacteriuria, pharmacy had the highest significant level of bacteriuria, with 23 (16.3%) prevalence followed by MLS, with 17 (12.0%) prevalence, and the other subsequent department. This report is in contrast with the study done by Enwerem et al.,[24] although the outcome is dependent on difference in the number of departments and sample size used for the study. A substantial number of pathogenic bacteria was found by bacteriological examination, with S. aureus accounting for the majority of isolates (54.97%), followed by Salmonella typhi (23.78%), E. coli (17.07%), and S. dysenteriae (4.24%). Given that S. aureus is one of the main causes of both symptomatic and asymptomatic infections, our results are in line with the larger epidemiological trend of bacterial pathogens that commonly cause UTIs.[24] Due to the possibility of antibiotic resistance, the high frequency of S. aureus calls for concern. The relative prevalence of S. aureus in this study emphasizes the significance of taking into account both Gram-positive and Gram-negative pathogens when examining urinary health in asymptomatic populations, even though E. coli and Salmonella are also frequently linked to gastrointestinal and urinary infections. Significant AMR is evident in the antibiotic susceptibility patterns found in this investigation, particularly among the isolates of S. aureus. The statistics reveal concerning rates of resistance to popular antibiotics, such as erythromycin (57.1% resistant), gentamicin (76.9% resistant), and amoxicillin-clavulanate (89% resistant). These high rates of resistance are in line with worries around the world regarding the abuse and overuse of antibiotics, which is causing MDROs to arise.[25] Given that S. aureus is a prevalent cause of skin infections and UTIs, which are commonly treated empirically with these antibiotics, this finding is particularly concerning.[26] Both Salmonella typhi and E. coli were shown to be resistant to routinely given medications such as CRO and CIP among the Gram-negative isolates. This is consistent with research demonstrating a growing pattern of cephalosporin and fluoroquinolone resistance in these organisms, which makes treatment choices more challenging.[27] Significantly, isolates of E. coli exhibited 100% resistance to CTX and ampiclox, indicating that these antibiotics may no longer be suitable first-line therapies in this population. The study’s AMR profiles highlight how urgent it is to update empirical treatment recommendations, with an emphasis on minimizing the overuse of antibiotics. More efficient management of antibiotic prescriptions is necessary since the high rates of resistance to gentamycin, CIP, and amoxicillin-clavulanate in S. aureus isolates indicate that these widely used antibiotics are losing their effectiveness. The study’s conclusions have important ramifications for clinical practice and public health at Madonna University and other comparable educational establishments. If neglected, ASB even in the absence of obvious clinical symptoms can have long-term effects, particularly when resistant bacterial strains are present. These results point to the necessity of more frequent student screenings for bacterial colonizations and UTIs, especially in light of the growing prevalence of AMR. To investigate the underlying risk factors linked to urine anomalies in asymptomatic persons, more research is necessary. The prevalence of UTIs and the emergence of AMR may be influenced by variables such as sexual activity, hygienic habits, history of antibiotic use, and socioeconomic position.[28] The long-term dangers associated with ASB might be better understood by longitudinal studies that monitor the development of subclinical infections into symptomatic illness. To discourage self-medication, encourage the prudent use of antibiotics, and lessen the needless use of broad-spectrum antibiotics, educational institutions must also establish antimicrobial stewardship programs in light of the high prevalence of resistance. The development of resistant infections may also be avoided by teaching students about good hygiene and the value of prompt treatment for urine symptoms. Interpreting the study’s findings requires taking into account a number of limitations. Even while the sample size is somewhat representative of the student body, it is still too small to draw broad conclusions. Because the study only looked at male students, its conclusions cannot be applied to female students or the broader public. In addition, although though the dipstick urinalysis is a helpful screening method, it is not always effective in identifying all kinds of urinary infections or anomalies. Therefore, more reliable information would be obtained from a more thorough diagnostic method that includes sensitivity testing and urine culture. This study concludes by highlighting the prevalence of bacterial infections and asymptomatic urine anomalies among male undergraduate students. The need for better diagnostic screening and antimicrobial stewardship is highlighted by the high frequency of S. aureus and other infections as well as concerning patterns of antibiotic resistance.
CONCLUSION
This study concludes by highlighting the prevalence of bacterial infections and asymptomatic urine anomalies among male undergraduate students at Madonna University, Elele River State south-south, Nigeria. The need for better diagnostic screening and antimicrobial stewardship is highlighted by the high frequency of Staphylococcus aureus and other infections as well as concerning patterns of antibiotic resistance. Universities and healthcare practitioners are urged by these findings to give infection control measures top priority, teach students good hygiene habits, and create focused interventions to fight antibiotic resistance.
Recommendation
Based on the findings of this study, several key recommendations can be made to improve the health and well-being of the undergraduate male students particularly in relation to urinary health and the management of AMR. It is recommended that routine urinary screening be introduced as part of the regular health check-ups for students, especially at the beginning of each academic year. Screening for urinary abnormalities, including proteinuria, bilirubin, and urobilinogen, should be prioritized to identify asymptomatic individuals at risk for UTIs or other renal disorders. Early detection of bacteriuria or other urine abnormalities could prevent the progression of infections and reduce the risk of developing more serious conditions. The findings of significant AMR to commonly prescribed antibiotics, particularly among S. aureus and E. coli isolates, underscore the need for implementing antimicrobial stewardship programs on campus. These programs should focus on educating students, faculty, and healthcare providers about the dangers of antibiotic misuse and overuse. Policies that limit the use of broad-spectrum antibiotics and encourage the appropriate use of targeted therapies based on sensitivity testing should be enforced. Furthermore, students should be educated about the importance of completing prescribed courses of antibiotics and avoiding self-medication. The university should collaborate with local health authorities and clinical laboratories to facilitate the timely diagnosis and treatment of bacterial infections among students. Collaboration could involve setting up referral systems for more complex cases, improving access to diagnostic tests, and ensuring that students have access to appropriate antibiotics when necessary. This partnership can also aid in the implementation of public health campaigns to raise awareness about AMR and the importance of infection control. Longitudinal studies should be conducted to track the progression of ASB in university students over time, as well as to monitor the emergence of AMR. Research on the factors that contribute to the high prevalence of urinary abnormalities and bacterial infections in this population would provide valuable data for improving preventive health strategies. In particular, studies examining lifestyle factors such as diet, hydration, physical activity, and sexual behavior could help identify modifiable risk factors for UTIs and other urinary conditions.
Acknowledgment:
The authors acknowledge the support from the Department of Pharmaceutical microbiology and Biotechnology, Faculty of Pharmacy, Madonna University, Elele, Rivers State, Nigeria.
Ethical approval:
The research was approved by the Madonna University Elele River State, Nigeria Institutional Review Board, number MAU/DRC/HD/E/PHARM/2024/056, dated 3rd November, 2024.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Asymptomatic bacteriuria: When to screen and when to treat. Infect Dis Clin North Am. 2019;33:595-606.
- [Google Scholar]
- Asymptomatic bacteriuria in healthy young male students: An underreported condition. J Clin Urol. 2021;14:91-5.
- [Google Scholar]
- Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice. Asymptomatic bacteriuria in adults. N Engl J Med. 2019;370:1837-44.
- [Google Scholar]
- Prevalence, antimicrobial susceptibility profile and predictors of asymptomatic bacteriuria among pregnant women in Adigrat general hospital, Northern Ethiopia. BMC Res Notes. 2018;11:740-45.
- [CrossRef] [Google Scholar]
- Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269-84.
- [CrossRef] [PubMed] [Google Scholar]
- Mathematical formulae for sample size determination Wuhan: Scientific Research Publishing; 1967. p. :1-4.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [CrossRef] [PubMed] [Google Scholar]
- Carbapenem-resistant Enterobacteriaceae posing a dilemma in effective healthcare delivery. Antibiotics (Basel). 2019;8:156.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infection and asymptomatic bacteriuria in older adults. Infect Dis Clin North Am. 2013;31:673-88.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of antibiotic resistant bacteria in Nigerian fermented food condiments. J Biol Life Sci. 2020;11:110-20.
- [CrossRef] [Google Scholar]
- Bed linen: A reservoir of antibiotic-resistant bacterial pathogens. J Curr Biomed Res. 2022;2:311-6.
- [CrossRef] [Google Scholar]
- The bacteriology and its virulence factors in neonatal infections: Threats to child survival strategies. J Pathog. 2018;2018:4801247.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-drug resistant acute otitis media amongst children attending out-patient clinic in Chukwuemeka Odumegwu Ojukwu university teaching hospital, Awka, South-East Nigeria. Adv Microbiol. 2016;6:495-501.
- [CrossRef] [Google Scholar]
- Asymptomatic urinary tract infection among school children in rural area of Ebonyi State. Ann Biol Res. 2012;3:2353-6.
- [Google Scholar]
- Prevalence of asymptomatic bacteriuria among pre-school children in Nnewi, South-East Nigeria. Niger J Pad. 2013;40:278-83.
- [Google Scholar]
- Asymptomatic bacteriuria among secondary school students in Benin City, Nigeria. J Public Health Epidemiol. 2013;5:66-9.
- [Google Scholar]
- Community acquired urinary tract infection prevalence in a tertiary institution based in Evbuobanosa, Edo State, Nigeria. Glob J Med Res Interdiscip. 2015;15:54-64.
- [Google Scholar]
- Microbiological profile of asymptomatic bacteriuria in pregnancy. Int J Reprod Contracept Obstet Gynecol. 2017;6:1352-61.
- [CrossRef] [Google Scholar]
- Antibiogram profile of uropathogens isolated at Bahir Dar regional health research laboratory centre, Northwest Ethiopia. Pan Afr Med J. 2017;26:134.
- [CrossRef] [PubMed] [Google Scholar]
- Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J Antimicrob Chemother. 2007;59:246-53.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors for asymptomatic bacteriuria among university students. BMC Urol. 2018;18:19.
- [Google Scholar]
- Assessment of bacteriological and antibiogram of uropathogens among students in the faculty of health sciences, Imo State University, Owerri. Int J Pathog Res. 2022;9:38-45.
- [CrossRef] [Google Scholar]
- Bacteriological profile and antimicrobial susceptibility pattern of urinary pathogens among Nigerian undergraduates. J Infect Dev Ctries. 2020;14:1065-72.
- [Google Scholar]
- Antibiotic resistance in Staphylococcus aureus isolates from Nigerian hospitals. Int J Antimicrob Agents. 2017;49:147-53.
- [Google Scholar]
- Antimicrobial resistance in Escherichia coli and Salmonella typhi in Nigerian hospitals: The need for proper antibiotic stewardship. J Afr Health Sci. 2019;19:2292-300.
- [Google Scholar]
- Antimicrobial resistance among uropathogens in the Asia-pacific region: A systematic review. JAC Antimicrob Resist. 2012;3:dlab003.
- [CrossRef] [PubMed] [Google Scholar]







