Translate this page into:
Anti-tuberculosis treatment-induced hepatotoxicity in a post-operative setting

*Corresponding author: Bincy Charley, Department of Clinical Pharmacy, Believers Church Medical College Hospital, Thiruvalla, Kerala, India. bincycharley0399@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Charley B, Mary Roy R, Philip S. Anti-tuberculosis treatment-induced hepatotoxicity in a post-operative setting. Glob J Health Sci Res. 2024;2:56-8. doi: 10.25259/GJHSR_24_2023
Abstract
The most frequent cause of infectious disease-related mortality globally is tuberculosis (TB), a multisystemic disease with a wide range of presentations and symptoms. Around 12% of extrapulmonary TB cases and 1–3% of all TB cases are caused by abdominal TB. The issue of antituberculosis drug-induced liver injury (ATLI), which has long been a challenge in the management of TB infection, is one of increased significance. The major steps to reduce the occurrence of ATLI are to identify the possible risk factors of hepatotoxicity and to provide an appropriate weight-based anti-tuberculosis treatment (ATT). This case report clarifies the significance of appropriate weight-based dosing of ATT, particularly in a post-operative environment as evidenced in this instance where the patient experienced considerable post-operative weight loss.
Keywords
Tuberculosis
Chemical and drug induced liver injury
Communicable diseases
Adverse drug reactions
Case report
INTRODUCTION
Tuberculosis (TB) continues to be the leading cause of death from an infectious disease among adults worldwide, with an incidence of 10 million.[1] The abdomen is involved in 11% of extrapulmonary TB patients.[2] Abdominal TB accounts for approximately 12% of extrapulmonary TB cases and 1–3% of total TB cases.[3,4] Drug-induced liver injury (DILI) is a growing issue that has long been a concern in the treatment of TB infection. DILI can occur with the majority of the currently recommended regimens for treating latent TB infection, including isoniazid or rifampicin. This is also true for two-drug regimens of pyrazinamide plus ethambutol or a fluoroquinolone used to treat contacts of multidrug-resistant (MDR) TB cases.[5] In this case report, we present the clinical scenario of anti-tuberculosis treatment (ATT) induced hepatitis in a 26-year-old male patient.
CASE REPORT
A 26-year-old gentleman presented to the Emergency Room with complaints of severe colicky abdominal pain of 3-day duration and multiple episodes of vomiting. He was a known case of hydrocephalus, seizures, and kyphoscoliosis. He was wheelchair bound and dependent on his family for his daily activities. He had been taking Levetiracetam 500 mg BD orally for management of seizures.
He was diagnosed to have intestinal obstruction for which he underwent an emergency laparotomy. Intraoperatively, there were numerous peritoneal deposits on the visceral and parietal peritoneum causing obstruction [Figure 1]. Along with adhesiolysis biopsy was taken from small bowel mesentery, parietal peritoneum, and omentum. The reports showed granulomatous inflammation with central caseous necrosis, favoring abdominal TB. He had a prolonged recovery period of 2 weeks whereby he was initially kept nil per orally and later slowly advanced to a liquid diet as tolerated.

- Intraoperative image of abdominal tuberculosis.
In view of abdominal TB, he was started on treatment with antitubercular drugs by the local primary health center after notifying the district TB center. The patient’s weight was assessed to be 46 kg after a weight examination, which included the weight of the wheelchair since it was difficult to weigh him otherwise as he was wheel chair bound. According to this, he was started on Rifampicin 450 mg (150 mg × 3 tablets), Isoniazid 225 mg (75 × 3), Pyrazinamide 1220 mg (400 × 3), and Ethambutol 825 mg (275 × 3 doses) as per the Revised National TB Control Program (RNTCP) of the National TB Elimination Program [Table 1] run by the Government of India.[6]
| S. No. | Anti-TB drug name | Initial dose (in mg) |
|---|---|---|
| 1. | Rifampicin | 450 mg (150 mg×3 tablets) |
| 2. | Isoniazid | 225 mg (75 mg×3 tablets) |
| 3. | Pyrazinamide | 1220 mg (400 mg×3 tablets) |
| 4. | Ethambutol | 825 mg (275 mg×3 tablets) |
RNTCP: Revised National Tuberculosis Control Program, ATT: Anti-tuberculosis treatment, TB: Tuberculosis
After about 2 weeks of ATT, he presented with complaints of abdominal pain and recurrent episodes of vomiting. He was admitted for further evaluation and management. His routine investigations showed deranged liver function test (LFT) and hyponatremia. The total bilirubin and indirect bilirubin were raised as well at the serum glutamic-pyruvic transaminase, serum glutamic-oxaloacetic transaminase, and alkaline phosphatase (ALP). He was diagnosed as having ATT-induced hepatotoxicity and reported to the District TB center. A multidisciplinary consultation was done involving the clinical pharmacist, gastroenterology, and community medicine departments to find the cause and treatment for the same. It was found that the ATT regimen was started based on his initially assessed weight of 46 kg whereas the actual weight of the patient was only 31 kg (excluding the weight of the wheelchair). After surgery, he had lost 8 kg with a current weight of 23 kg. It was decided to stop ATT and start each medicine as ATT challenge progressively based on his current weight as per [Figure 2]. His LFT normalized 1 week after stopping ATT. He was reintroduced to ATT as per [Table 1] and later tolerated the intensive phase of ATT well.
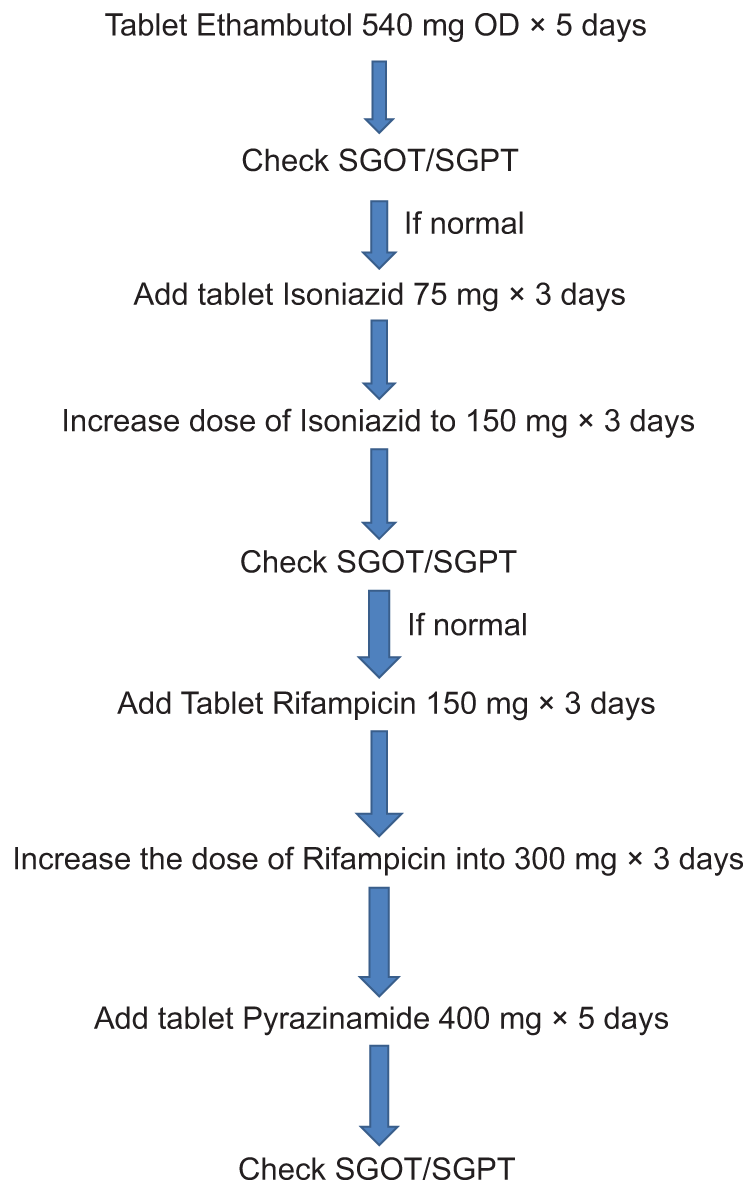
- Anti-tuberculosis treatment reintroduction regimen.
DISCUSSION
The principle behind a daily regimen for TB treatment is to give patients daily fixed-dose combinations of the first-line anti-TB medications in the correct weight bands. The treatment will last nine to 12 months in total. The intense phase lasts for 3 months with a possible extension to a maximum of 6 months. The continuation phase has a fixed time limit of 6 months.[6] Identification of the risk factors for hepatotoxicity is essential to prevent it during ATT and improve patient outcomes. One lesser-known risk factor, as seen in this case, is failing to prescribe the recommended dose of ATT medications based on the patient’s current weight.
Conventionally, risk stratification is done for the following groups of patients: Those who consume alcohol, those taking concurrent hepatotoxic medications, those with chronic hepatitis B or C, human immunodeficiency virus, and those with raised baseline transaminase values.[7] Our case report expounds a case for accurate weight-based dosing of ATT. Special attention may be given to post-operative patients since they usually have significant loss of weight after a major surgery. In this case, hepatotoxicity was managed conservatively by stopping ATT and correcting electrolyte imbalance. Adjuvant therapies are of doubtful benefit and hence not used.[8]
The mechanism underlying DILI is not well understood and there is still no single biomarker or diagnostic tool to unequivocally diagnose DILI. We have used the international consensus criteria (alanine aminotransferase, conjugated bilirubin, or ALP >2 × the upper limit of normal) to diagnose ATT-induced hepatotoxicity in this case.[9]
As stated in a study conducted in 2022 regarding the incidence of antituberculosis drug-induced liver injury (ATLI), the incidence curve for ATLI showed a clear upward trend worldwide.[10] Identifying various risk factors as shown in this report for the same may help in decreasing the incidence of ATLI especially accurate weight-based dosing as seen in this instance where the patient had significant loss of weight postoperatively.
CONCLUSION
Identifying the potential risk factors of hepatotoxicity and providing an appropriate weight based ATT are the key measures to decrease the incidence of ATLI especially in a perioperative setting. Dosage adjustment in the intensive phase helps overcome the complications of liver injury associated with these drugs. Withdrawal of the drugs followed by reintroduction in a weight based manner, once the LFT is normal, helps achieve tolerance to the drugs.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 2003;5:273-8.
- [CrossRef] [PubMed] [Google Scholar]
- Extrapulmonary tuberculosis in the United States. Am J Epidemiol. 1979;109:205-17.
- [CrossRef] [PubMed] [Google Scholar]
- An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935-52.
- [CrossRef] [PubMed] [Google Scholar]
- Ministry of Health and Family Welfare-Government of India. Home: Central TB Division. Available from: https://www.tbcindia.gov.in [Last accessed on 2023 Jan 16]
- [Google Scholar]
- A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob Agents Chemother. 2013;57:1685-90.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive use of hepatoprotectors yields limited efficacy on the liver toxicity of anti-tuberculosis agents in a large cohort of Chinese patients. J Gastroenterol Hepatol. 2015;30:540-5.
- [CrossRef] [PubMed] [Google Scholar]
- Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and temporal trend of antituberculosis drug-induced liver injury: A systematic review and meta-analysis. J Trop Med. 2022;2022:8266878.
- [CrossRef] [PubMed] [Google Scholar]







